Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury
- PMID: 32460853
- PMCID: PMC7251891
- DOI: 10.1186/s13287-020-01719-2
Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury
Abstract
Background: Exosomes derived from mesenchymal stem cells (MSC-exos) have been demonstrated with great potential in the treatment of multiple human diseases including acute kidney injury (AKI) by virtue of their intrinsic cargoes. However, there are major challenges of low yield and the lack of an established biomanufacturing platform to efficiently produce MSC-exos, thereby limiting their therapeutic application. Here, we aimed to establish a novel strategy to produce MSC-exos with a hollow fiber bioreactor-based three-dimensional (3D) culture system and evaluate the therapeutic efficacy of 3D-exosomes (3D-exos) on AKI.
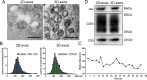
Methods: Mesenchymal stem cells (MSCs) were isolated from fresh human umbilical cord and cultured in two-dimensional (2D) flasks. 2 × 108 MSCs were inoculated into the hollow fiber bioreactor for 3D culture. The culture supernatants were collected every 1 or 2 days for isolating exosomes. Exosomes from 2D (2D-exos) and 3D cultures were characterized by transmission electron microscopy, nanoparticle tracking analysis, and western blotting analysis of exosome markers. The yield of exosomes from 2 × 108 MSCs seeded in 2D and 3D culture system was compared, based on protein quantification. The therapeutic efficacy of 2D-exos and 3D-exos was investigated in a murine model of cisplatin-induced AKI in vivo and in vitro.
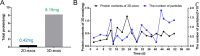
Results: 3D culture did not significantly change the surface markers of MSCs, as well as the morphology, size, and exosomal markers of 3D-exos when compared to those of 2D-exos. Compared with conventional 2D culture, the 3D culture system increased total exosome production up to 19.4-fold. 3D-exos were more concentrated in the harvested supernatants (15.5-fold) than 2D-exos, which led to a higher exosome collection efficiency of 3D culture system. In vivo, both 2D-exos and 3D-exos significantly alleviated cisplatin-induced murine AKI evidenced by improved renal function, attenuated pathological changes of renal tubules, reduced inflammatory factors, and repressed T cell and macrophage infiltration. Impressively, 3D-exos were more effective than 2D-exos. Moreover, 3D-exos were taken up by tubular epithelial cells (TECs) with improved efficiency, thereby exhibiting superior anti-inflammatory effect and improved viability of TECs in vitro.
Conclusions: In summary, our findings demonstrate that the hollow fiber 3D culture system provides an efficient strategy for the continuous production of MSC-exos which has enhanced therapeutic potential for cisplatin-induced AKI.
Keywords: Acute kidney injury; Exosomes; Mesenchymal stem cell; Three-dimensional culture.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Gazdic M, Volarevic V, Arsenijevic N, et al. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep. 2015;11(2):280–287. - PubMed
-
- Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. - PubMed
-
- Lindsay JO, Allez M, Clark M, et al. Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol Hepatol. 2017;2(6):399–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

