Characterization of splenic MRC1hiMHCIIlo and MRC1loMHCIIhi cells from the monocyte/macrophage lineage of White Leghorn chickens
- PMID: 32460863
- PMCID: PMC7251834
- DOI: 10.1186/s13567-020-00795-9
Characterization of splenic MRC1hiMHCIIlo and MRC1loMHCIIhi cells from the monocyte/macrophage lineage of White Leghorn chickens
Abstract
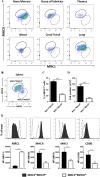
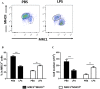
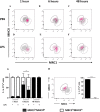
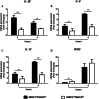
Monocytes/macrophages, which are found in a variety of organs, maintain tissue homeostasis at a steady state and act as the first line of defence during pathogen-induced inflammation in the host. Most monocyte/macrophage lineage studies in chickens have been largely performed using cell lines, while few studies using primary cells have been conducted. In the present study, the phenotypic and functional characteristics of splenic monocyte/macrophage lineage cells during steady state and inflammatory conditions were examined. Splenic monocyte/macrophage lineage cells could be identified as MRC1loMHCIIhi and MRC1hiMHCIIlo cells based on their surface expression of MRC1 and MHCII. In the steady state, MRC1loMHCIIhi cells were more frequently found among MRC1+ cells. MRC1loMHCIIhi cells expressed a higher number of antigen-presenting molecules (MHCII, MHCI, and CD80) than MRC1hiMHCIIlo cells. In contrast, MRC1hiMHCIIlo cells showed better phagocytic and CCR5-dependent migratory properties than MRC1loMHCIIhi cells. Furthermore, MRC1hiMHCIIlo cells infiltrated the spleen in vivo and then became MRC1loMHCIIhi cells. During lipopolysaccharide (LPS)-induced inflammatory conditions that were produced via intraperitoneal (i.p.) injection, the proportion and absolute number of MRC1hiMHCIIlo cells were increased in the spleen. Uniquely, inflammation induced the downregulation of MHCII expression in MRC1hiMHCIIlo cells. The major source of inflammatory cytokines (IL-1β, IL-6, and IL-12) was MRC1loMHCIIhi cells. Furthermore, MRC1hiMHCIIlo cells showed greater bactericidal activity than MRC1loMHCIIhi cells during LPS-induced inflammation. Collectively, these results suggest that two subsets of monocyte/macrophage lineage cells exist in the chicken spleen that have functional differences.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
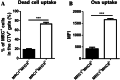



References
-
- Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302:1560–1563. - PubMed
-
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

