Pulmonary capillary haemangiomatosis: a distinct entity?
- PMID: 32461209
- PMCID: PMC9488541
- DOI: 10.1183/16000617.0168-2019
Pulmonary capillary haemangiomatosis: a distinct entity?
Abstract
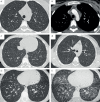
Pulmonary capillary haemangiomatosis (PCH) is a rare and incompletely understood histopathological finding characterised by abnormal capillary proliferation within the alveolar interstitium, which has long been noted to share many overlapping features with pulmonary veno-occlusive disease (PVOD). But are PCH and PVOD distinct entities that occur in isolation, or are they closely intertwined manifestations along a spectrum of the same disease? The classic clinical features of both PCH and PVOD include signs and symptoms related to pulmonary hypertension, hypoxaemia, markedly impaired diffusion capacity of the lung and abnormal chest imaging with ground glass opacities, septal lines and lymphadenopathy. In recent years, increasing evidence suggests that the clinical presentation, histopathological features, genetic substrate and pathobiological mechanisms of PCH and PVOD are overlapping and usually indistinguishable. The discovery of biallelic mutations in the eukaryotic translation initiation factor 2 α kinase 4 (EIF2AK4) gene in heritable PCH and PVOD greatly advanced our understanding of the overlapping nature of these conditions. Furthermore, recognition of PCH and PVOD-like changes in other pulmonary vascular diseases and in conditions that cause chronic pulmonary venous hyper-perfusion or hypertension suggests that PCH/PVOD may develop as a reactive process to various insults or injuries to the pulmonary vasculature, rather than being primary angiogenic disorders.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: J. Weatherald reports grants, personal fees and non-financial support from Janssen Inc. and Actelion, personal fees and non-financial support from Bayer, personal fees from Novartis, and grants from Alberta Lung Association Canadian Vascular Network, European Respiratory Society and Canadian Thoracic Society, outside the submitted work. Conflict of interest: P. Dorfmüller has nothing to disclose. Conflict of interest: F. Perros has nothing to disclose. Conflict of interest: M-R. Ghigna has nothing to disclose. Conflict of interest: B. Girerd has nothing to disclose. Conflict of interest: M. Humbert reports personal fees and non-financial support from Acceleron, grants and personal fees from Bayer and GSK, and personal fees from Actelion, Merck and United Therapeutics, outside the submitted work. Conflict of interest: D. Montani reports grants and personal fees from Actelion and Bayer, and personal fees from GSK Pfizer, MSD and Chiesi, outside the submitted work.
Figures






Comment in
References
-
- Pietra GG, Edwards WD, Kay JM, et al. . Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 1989; 80: 1198–1206. doi:10.1161/01.CIR.80.5.1198 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
