Frameshift mutations of YPEL3 alter the sensory circuit function in Drosophila
- PMID: 32461240
- PMCID: PMC7286299
- DOI: 10.1242/dmm.042390
Frameshift mutations of YPEL3 alter the sensory circuit function in Drosophila
Abstract
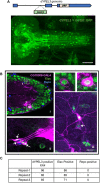
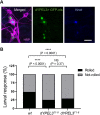
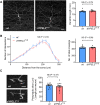
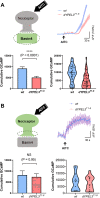
A frameshift mutation in Yippee-like (YPEL) 3 was recently found from a rare human disorder with peripheral neurological conditions including hypotonia and areflexia. The YPEL gene family is highly conserved from yeast to human, but its members' functions are poorly defined. Moreover, the pathogenicity of the human YPEL3 variant is completely unknown. We generated a Drosophila model of human YPEL3 variant and a genetic null allele of Drosophila homolog of YPEL3 (referred to as dYPEL3). Gene-trap analysis suggests that dYPEL3 is predominantly expressed in subsets of neurons, including larval nociceptors. Analysis of chemical nociception induced by allyl-isothiocyanate (AITC), a natural chemical stimulant, revealed reduced nociceptive responses in both dYPEL3 frameshift and null mutants. Subsequent circuit analysis showed reduced activation of second-order neurons (SONs) in the pathway without affecting nociceptor activation upon AITC treatment. Although the gross axonal and dendritic development of nociceptors was unaffected, the synaptic contact between nociceptors and SONs was decreased by the dYPEL3 mutations. Furthermore, expressing dYPEL3 in larval nociceptors rescued the behavioral deficit in dYPEL3 frameshift mutants, suggesting a presynaptic origin of the deficit. Together, these findings suggest that the frameshift mutation results in YPEL3 loss of function and may cause neurological conditions by weakening synaptic connections through presynaptic mechanisms.
Keywords: Pathogenicity; Rare mutation; Synaptic connection; YPEL3.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

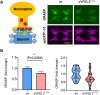
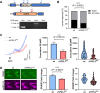
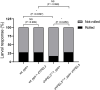
References
-
- Baker S. J. (2003). Small unstable apoptotic protein, an apoptosis-associated protein, suppresses proliferation of myeloid cells. Cancer Res. 63, 705-712. - PubMed
-
- Blaker-Lee A., Gupta S., McCammon J. M., De Rienzo G. and Sive H. (2012). Zebrafish homologs of genes within 16p11.2, a genomic region associated with brain disorders, are active during brain development, and include two deletion dosage sensor genes. Dis. Model. Mech. 5, 834-851. 10.1242/dmm.009944 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

