Two-year interim safety results of the 0.2 µg/day fluocinolone acetonide intravitreal implant for the treatment of diabetic macular oedema: the observational PALADIN study
- PMID: 32461262
- PMCID: PMC7907551
- DOI: 10.1136/bjophthalmol-2020-315984
Two-year interim safety results of the 0.2 µg/day fluocinolone acetonide intravitreal implant for the treatment of diabetic macular oedema: the observational PALADIN study
Abstract
Background: The 0.2 µg/day fluocinolone acetonide (FAc) implant delivers continuous, low-dose, intravitreal corticosteroid for the treatment of diabetic macular oedema (DMO). This ongoing, 3-year, observational clinical trial provides long-term, 'real-world' safety results for the FAc implant in DMO.
Methods: This 24-month interim analysis of a prospective, observational study investigated patients with DMO receiving the commercially available intravitreal 0.2 µg/day FAc implant. The primary outcome was incidence of intraocular pressure (IOP)-lowering procedures. Other IOP-related signals and their relationship to previous corticosteroid exposure, best-corrected visual acuity, central subfield thickness (CST), ocular adverse events and frequency of other treatments were also measured.
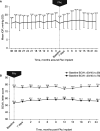
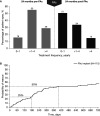
Results: Data were collected from 95 previously steroid-challenged patients (115 study eyes) for up to 36 months pre-FAc and 24 months post-FAc implant. Mean IOP for the overall population remained stable post-FAc compared with pre-FAc implant. IOP-related procedures remained infrequent (two IOP-lowering surgeries pre-FAc; two trabeculoplasties and four IOP-lowering surgeries post-FAc). Mean visual acuity was stable post-FAc (mean improvement of 1-3 letters) and fewer DMO treatments were required per year following FAc implant. Mean CST was significantly reduced at 24 months post-FAc implant (p<0.001) and the percentage of patients with CST ≤300 µm was significantly increased (p=0.041).
Conclusion: Few IOP-related procedures were reported during the 24 months post-FAc implant. Positive efficacy outcomes were noted after treatment, with stabilisation of vision and reduction in inflammation, demonstrated by CST. The FAc implant has a favourable benefit-risk profile in the management of DMO, especially when administered after a prior steroid challenge.
Trial registration number: NCT02424019.
Keywords: inflammation; intraocular pressure; macula; retina; treatment other.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SEM has received clinical trial research funds from Alimera Sciences, Chengdu Kanghong Biotechnology, Clearside Biomedical and Genentech. DFK has received research support from Adverum, Alimera Sciences, Allergan, EyePoint and Graybug Pharmaceuticals, received consultant fees from Adverum, Alimera Sciences, Allergan, BVI, EyePoint, Genentech, Mallinckrodt, Notal Vision, Novartis, Physician Recommended Neutriceuticals and Regeneron, and participated in speakers bureau for Alimera Sciences, Allergan, EyePoint, Genentech, Mallinckrodt, Notal Vision, Physician Recommended Neutriceuticals, Regeneron and Spark Therapeutics. DBR has received personal fees from Alimera Sciences, Allergan and Genentech. DE has received personal fees from Alimera Sciences, Allergan, Clearside, EyePoint, Genentech, Gyroscope, Kodiak, Network Eye, Notal Vision, Novartis and Regeneron, received research support from Alimera Sciences, Allergan, Chengdu, Clearside, DORC, Genentech, Gyroscope, Kodiak NGM, Mylan, Network Eye, Novartis, Ophthotech, Ophthea and Recens Medical and received equity from Boston Image Reading Center, Hemera Biopharmaceuticals and US Retina. NMH has received research support from Gemini, Gyroscope, Roche and Genentech, received consultant fees from Acucela, Alimera Sciences, Allegro, Allergan, Clearside, Genentech, Katalyst, Lineage Cell Therapeutics, Notal Vision, Novartis, Regeneron, and Spark, and has patents and stock ownership with Katalyst. SK and EW are employees of Alimera Sciences.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
