Comparison of Two Commercial Molecular Tests and a Laboratory-Developed Modification of the CDC 2019-nCoV Reverse Transcriptase PCR Assay for the Detection of SARS-CoV-2
- PMID: 32461287
- PMCID: PMC7383545
- DOI: 10.1128/JCM.00938-20
Comparison of Two Commercial Molecular Tests and a Laboratory-Developed Modification of the CDC 2019-nCoV Reverse Transcriptase PCR Assay for the Detection of SARS-CoV-2
Abstract
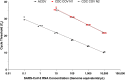
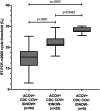
We compared the ability of 2 commercial molecular amplification assays (RealTime SARS-CoV-2 on the m2000 [abbreviated ACOV; Abbott] and ID Now COVID-19 [abbreviated IDNOW; Abbott]) and a laboratory-developed test (modified CDC 2019-nCoV reverse transcriptase PCR [RT-PCR] assay with RNA extraction by eMag [bioMérieux] and amplification on QuantStudio 6 or ABI 7500 real-time PCR system [abbreviated CDC COV]) to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in upper respiratory tract specimens. Discrepant results were adjudicated by medical record review. A total of 200 nasopharyngeal swab specimens in viral transport medium (VTM) were collected from symptomatic patients between 27 March and 9 April 2020. Results were concordant for 167 specimens (83.5% overall agreement), including 94 positive and 73 negative specimens. The ACOV assay yielded 33 additional positive results, 25 of which were also positive by the CDC COV assay but not by the IDNOW assay. In a follow-up evaluation, 97 patients for whom a dry nasal swab specimen yielded negative results by IDNOW had a paired nasopharyngeal swab specimen collected in VTM and tested by the ACOV assay; SARS-CoV-2 RNA was detected in 13 (13.4%) of these specimens. Medical record review deemed all discrepant results to be true positives. The IDNOW test was the easiest to perform and provided a result in the shortest time but detected fewer cases of COVID-19. The ACOV assay detected more cases of COVID-19 than the CDC COV or IDNOW assays.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; coronavirus; novel coronavirus.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. doi: 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC, Singapore Novel Coronavirus Outbreak Research Team. 2020. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323:1488–1494. doi: 10.1001/jama.2020.3204. - DOI - PMC - PubMed
-
- Bernard Stoecklin S, Rolland P, Silue Y, Mailles A, Campese C, Simondon A, Mechain M, Meurice L, Nguyen M, Bassi C, Yamani E, Behillil S, Ismael S, Nguyen D, Malvy D, Lescure FX, Georges S, Lazarus C, Tabai A, Stempfelet M, Enouf V, Coignard B, Levy-Bruhl D, Investigation T. 2020. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill 25:pii=2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. - DOI - PMC - PubMed
-
- Spiteri G, Fielding J, Diercke M, Campese C, Enouf V, Gaymard A, Bella A, Sognamiglio P, Sierra Moros MJ, Riutort AN, Demina YV, Mahieu R, Broas M, Bengner M, Buda S, Schilling J, Filleul L, Lepoutre A, Saura C, Mailles A, Levy-Bruhl D, Coignard B, Bernard-Stoecklin S, Behillil S, van der Werf S, Valette M, Lina B, Riccardo F, Nicastri E, Casas I, Larrauri A, Salom Castell M, Pozo F, Maksyutov RA, Martin C, Van Ranst M, Bossuyt N, Siira L, Sane J, Tegmark-Wisell K, Palmerus M, Broberg EK, Beaute J, Jorgensen P, Bundle N, Pereyaslov D, Adlhoch C, Pukkila J, Pebody R, Olsen S, et al. 2020. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill 25:2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

