Endogenous TLR2 ligand embedded in the catalytic region of human cysteinyl-tRNA synthetase 1
- PMID: 32461342
- PMCID: PMC7254149
- DOI: 10.1136/jitc-2019-000277
Endogenous TLR2 ligand embedded in the catalytic region of human cysteinyl-tRNA synthetase 1
Abstract
Background: The generation of antigen-specific cytotoxic T lymphocyte (CTL) responses is required for successful cancer vaccine therapy. In this regard, ligands of Toll-like receptors (TLRs) have been suggested to activate adaptive immune responses by modulating the function of antigen-presenting cells (APCs). Despite their therapeutic potential, the development of TLR ligands for immunotherapy is often hampered due to rapid systemic toxicity. Regarding the safety concerns of currently available TLR ligands, finding a new TLR agonist with potent efficacy and safety is needed.
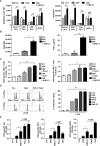
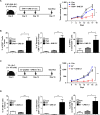
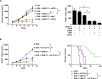
Methods: A unique structural domain (UNE-C1) was identified as a novel TLR2/6 in the catalytic region of human cysteinyl-tRNA synthetase 1 (CARS1) using comprehensive approaches, including RNA sequencing, the human embryonic kidney (HEK)-TLR Blue system, pull-down, and ELISA. The potency of its immunoadjuvant properties was analyzed by assessing antigen-specific antibody and CTL responses. In addition, the efficacy of tumor growth inhibition and the presence of the tumor-infiltrating leukocytes were evaluated using E.G7-OVA and TC-1 mouse models. The combined effect of UNE-C1 with an immune checkpoint inhibitor, anti-CTLA-4 antibody, was also evaluated in vivo. The safety of UNE-C1 immunization was determined by monitoring splenomegaly and cytokine production in the blood.
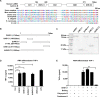
Results: Here, we report that CARS1 can be secreted from cancer cells to activate immune responses via specific interactions with TLR2/6 of APCs. A unique domain (UNE-C1) inserted into the catalytic region of CARS1 was determined to activate dendritic cells, leading to the stimulation of robust humoral and cellular immune responses in vivo. UNE-C1 also showed synergistic efficacy with cancer antigens and checkpoint inhibitors against different cancer models in vivo. Further, the safety assessment of UNE-C1 showed lower systemic cytokine levels than other known TLR agonists.
Conclusions: We identified the endogenous TLR2/6 activating domain from human cysteinyl-tRNA synthetase CARS1. This novel TLR2/6 ligand showed potent immune-stimulating activity with little toxicity. Thus, the UNE-C1 domain can be developed as an effective immunoadjuvant with checkpoint inhibitors or cancer antigens to boost antitumor immunity.
Keywords: adjuvants, immunological; immunology; oncology; vaccination.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
