Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10
- PMID: 32461349
- PMCID: PMC7254133
- DOI: 10.1136/jitc-2020-000599
Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10
Abstract
Background: Interleukin-15 (IL-15) promotes growth and activation of cytotoxic CD8+ T and natural killer (NK) cells. Bioactive IL-15 is produced in the body as a heterodimeric cytokine, comprising the IL-15 and IL-15 receptor alpha chains (hetIL-15). Several preclinical models support the antitumor activity of hetIL-15 promoting its application in clinical trials.
Methods: The antitumor activity of hetIL-15 produced from mammalian cells was tested in mouse tumor models (MC38 colon carcinoma and TC-1 epithelial carcinoma). The functional diversity of the immune infiltrate and the cytokine/chemokine network within the tumor was evaluated by flow cytometry, multicolor immunohistochemistry (IHC), gene expression profiling by Nanostring Technologies, and protein analysis by electrochemiluminescence and ELISA assays.
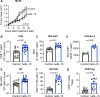
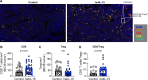
Results: hetIL-15 treatment resulted in delayed primary tumor growth. Increased NK and CD8+ T cell tumoral infiltration with an increased CD8+/Treg ratio were found by flow cytometry and IHC in hetIL-15 treated animals. Intratumoral NK and CD8+ T cells showed activation features with enhanced interferon-γ (IFN-γ) production, proliferation (Ki67+), cytotoxic potential (Granzyme B+) and expression of the survival factor Bcl-2. Transcriptomics and proteomics analyses revealed complex effects on the tumor microenvironment triggered by hetIL-15 therapy, including increased levels of IFN-γ and XCL1 with intratumoral accumulation of XCR1+IRF8+CD103+ conventional type 1 dendritic cells (cDC1). Concomitantly, the production of the chemokines CXCL9 and CXCL10 by tumor-localized myeloid cells, including cDC1, was boosted by hetIL-15 in an IFN-γ-dependent manner. An increased frequency of circulating CXCR3+ NK and CD8+ T cells was found, suggesting their ability to migrate toward the tumors following the CXCL9 and CXCL10 chemokine gradient.
Conclusions: Our results show that hetIL-15 administration enhances T cell entry into tumors, increasing the success rate of immunotherapy interventions. Our study further supports the incorporation of hetIL-15 in tumor immunotherapy approaches to promote the development of antitumor responses by favoring effector over regulatory cells and by promoting lymphocyte and DC localization into tumors through the modification of the tumor chemokine and cytokine milieu.
Keywords: CD8-positive T-lymphocytes; T-lymphocytes; cytokines; dendritic cells; immunotherapy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: CB, AV, BKF and GNP are inventors on US Government-owned patents related to hetIL-15.
Figures





References
-
- Naito Y, Saito K, Shiiba K, et al. . Cd8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998;58:3491–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
