Tumor ablation plus co-administration of CpG and saponin adjuvants affects IL-1 production and multifunctional T cell numbers in tumor draining lymph nodes
- PMID: 32461350
- PMCID: PMC7254152
- DOI: 10.1136/jitc-2020-000649
Tumor ablation plus co-administration of CpG and saponin adjuvants affects IL-1 production and multifunctional T cell numbers in tumor draining lymph nodes
Abstract
Background: Tumor ablation techniques, like cryoablation, are successfully used in the clinic to treat tumors. The tumor debris remaining in situ after ablation is a major antigen depot, including neoantigens, which are presented by dendritic cells (DCs) in the draining lymph nodes to induce tumor-specific CD8+ T cells. We have previously shown that co-administration of adjuvants is essential to evoke strong in vivo antitumor immunity and the induction of long-term memory. However, which adjuvants most effectively combine with in situ tumor ablation remains unclear.
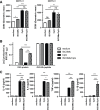
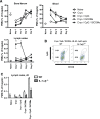
Methods and results: Here, we show that simultaneous administration of cytidyl guanosyl (CpG) with saponin-based adjuvants following cryoablation affects multifunctional T-cell numbers and interleukin (IL)-1 induced polymorphonuclear neutrophil recruitment in the tumor draining lymph nodes, relative to either adjuvant alone. The combination of CpG and saponin-based adjuvants induces potent DC maturation (mainly CpG-mediated), antigen cross-presentation (mainly saponin-based adjuvant mediated), while excretion of IL-1β by DCs in vitro depends on the presence of both adjuvants. Most strikingly, CpG/saponin-based adjuvant exposed DCs potentiate antigen-specific T-cell proliferation resulting in multipotent T cells with increased capacity to produce interferon (IFN)γ, IL-2 and tumor necrosis factor-α in vitro. Also in vivo the CpG/saponin-based adjuvant combination plus cryoablation increased the numbers of tumor-specific CD8+ T cells showing enhanced IFNγ production as compared with single adjuvant treatments.
Conclusions: Collectively, these data indicate that co-injection of CpG with saponin-based adjuvants after cryoablation induces an increased amount of tumor-specific multifunctional T cells. The combination of saponin-based adjuvants with toll-like receptor 9 adjuvant CpG in a cryoablative setting therefore represents a promising in situ vaccination strategy.
Keywords: CD8-positive T-lymphocytes; adaptive immunity; adjuvants, immunologic; dendritic cells; immunomodulation.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
