CYLD-mutant cylindroma-like basaloid carcinoma of the anus: a genetically and morphologically distinct class of HPV-related anal carcinoma
- PMID: 32461623
- PMCID: PMC7685972
- DOI: 10.1038/s41379-020-0584-2
CYLD-mutant cylindroma-like basaloid carcinoma of the anus: a genetically and morphologically distinct class of HPV-related anal carcinoma
Abstract
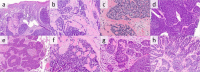
Rare reports of anal carcinoma (AC) describe histologic resemblance to cutaneous cylindroma, but mutations in the tumor suppressor CYLD, the gene responsible for familial and sporadic cylindromas, have not been systematically investigated in AC. Here, we investigate CYLD-mutant AC, focusing on molecular correlates of distinct histopathology. Comprehensive genomic profiling (hybrid-capture-based DNA sequencing) was performed on 574 ACs, of which 75 unique cases (13%) harbored a CYLD mutation. Clinical data, pathology reports, and histopathology were reviewed for each CYLD-mutant case. The spectrum of CYLD mutations included truncating (n = 50; 67%), homozygous deletion (n = 10; 13%), missense (n = 16; 21%), and splice-site (n = 3; 4%) events. Compared with CYLD-wildtype AC (n = 499), CYLD-mutant ACs were significantly enriched for females (88% vs. 67%, p = 0.0001), slightly younger (median age 59 vs. 61 years, p = 0.047), and included near-universal detection of high-risk HPV sequences (97% vs. 88%, p = 0.014), predominantly HPV16 (96%). The CYLD-mutant cohort also showed significantly lower tumor mutational burden (TMB; median 2.6 vs. 5.2 mut/Mb, p < 0.00001) and less frequent alterations in PIK3CA (13% vs. 31%, p = 0.0015). On histopathologic examination, 73% of CYLD-mutant AC (55/75 cases) showed a striking cylindroma-like histomorphology, composed of aggregates of basaloid cells surrounded by thickened basement membranes and containing characteristic hyaline globules, while only 8% of CYLD-wildtype tumors (n = 34/409) contained cylindroma-like hyaline globules (p < 0.0001). CYLD-mutant carcinomas with cylindroma-like histomorphology (n = 55) showed significantly lower TMB compared with CYLD-mutant cases showing basaloid histology without the distinctive hyaline globules (n = 14) (median 1.7 vs. 4.4 mut/Mb, p = 0.0058). Only five CYLD-mutant cases (7%) showed nonbasaloid conventional squamous cell carcinoma histology (median TMB = 5.2 mut/Mb), and a single CYLD-mutant case showed transitional cell carcinoma-like histology. Within our cohort of ACs, CYLD mutations characterize a surprisingly large subset (13%), with distinct clinical and genomic features and, predominantly, a striking cylindroma-like histopathology, representing a genotype-phenotype correlation which may assist in classification of AC.
Conflict of interest statement
EAW, MM, RS, DCP, ESS, BA, JV, JAE, JSR, SHR, ACH, and JYT are employees of Foundation Medicine, Inc., a wholly owned subsidiary of Roche Holdings, Inc. and Roche Finance Ltd, and these employees have equity interest in an affiliate of these Roche entities. KJW receives research funding from Novo Nordisk for studies unrelated to cancer. JC, PJP, BJG, and MCM declare no conflict of interest.
Figures


Similar articles
-
CYLD-mutated anal squamous cell carcinoma: An uncommon entity associated with cylindroma-like morphology and adverse clinical features.Hum Pathol. 2025 Mar;157:105765. doi: 10.1016/j.humpath.2025.105765. Epub 2025 Apr 1. Hum Pathol. 2025. PMID: 40180056
-
CYLD mutation characterizes a subset of HPV-positive head and neck squamous cell carcinomas with distinctive genomics and frequent cylindroma-like histologic features.Mod Pathol. 2021 Feb;34(2):358-370. doi: 10.1038/s41379-020-00672-y. Epub 2020 Sep 5. Mod Pathol. 2021. PMID: 32892208 Free PMC article.
-
Phenotype variability in tumor disorders of the skin appendages associated with mutations in the CYLD gene.Arch Dermatol Res. 2018 Sep;310(7):599-606. doi: 10.1007/s00403-018-1848-2. Epub 2018 Jul 4. Arch Dermatol Res. 2018. PMID: 29974194
-
Cylindroma of the breast with CYLD gene mutation: a case report and review of the literature.Mol Biol Rep. 2023 Aug;50(8):7133-7139. doi: 10.1007/s11033-023-08606-y. Epub 2023 Jun 30. Mol Biol Rep. 2023. PMID: 37389703 Free PMC article. Review.
-
Inherited cylindromas: lessons from a rare tumour.Lancet Oncol. 2015 Sep;16(9):e460-e469. doi: 10.1016/S1470-2045(15)00245-4. Lancet Oncol. 2015. PMID: 26370355 Review.
Cited by
-
CYLD in health and disease.Dis Model Mech. 2023 Jun 1;16(6):dmm050093. doi: 10.1242/dmm.050093. Epub 2023 Jun 30. Dis Model Mech. 2023. PMID: 37387450 Free PMC article. Review.
-
Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors.Cancers (Basel). 2022 Jan 18;14(3):476. doi: 10.3390/cancers14030476. Cancers (Basel). 2022. PMID: 35158743 Free PMC article. Review.
-
Molecular characterization of squamous cell carcinoma of the anal canal.J Gastrointest Oncol. 2021 Oct;12(5):2423-2437. doi: 10.21037/jgo-20-610. J Gastrointest Oncol. 2021. PMID: 34790403 Free PMC article.
-
CYLD Alterations in the Tumorigenesis and Progression of Human Papillomavirus-Associated Head and Neck Cancers.Mol Cancer Res. 2021 Jan;19(1):14-24. doi: 10.1158/1541-7786.MCR-20-0565. Epub 2020 Sep 3. Mol Cancer Res. 2021. PMID: 32883697 Free PMC article. Review.
-
Molecular and immunophenotypic characterization of anal squamous cell carcinoma reveals distinct clinicopathologic groups associated with HPV and TP53 mutation status.Mod Pathol. 2021 May;34(5):1017-1030. doi: 10.1038/s41379-020-00729-y. Epub 2021 Jan 22. Mod Pathol. 2021. PMID: 33483624
References
-
- Graham R. Anal squamous cell carcinoma. In: Goldblum J, Klimstra D, Lam A, editors. Digestive system tumours WHO classification of tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2019. pp. 205–7.
-
- Yang EJ. Human papilloma virus–associated squamous neoplasia of the lower anogenital tract. Surg Pathol Clin. 2019;12:263–79. - PubMed
-
- Ju JY, Stelow EB. Clinicopathologic features of anal and perianal squamous cell carcinomas and their relationship to human papillomavirus. Am J Surg Pathol. 2019;43:827–34. - PubMed
-
- Wong AK, Chan RC, Aggarwal N, Singh MK, Nichols WS, Bose S. Human papillomavirus genotypes in anal intraepithelial neoplasia and anal carcinoma as detected in tissue biopsies. Mod Pathol. 2010;23:144–50. - PubMed
-
- Klotz RG, Pamukcoclu T, Souilliard DH. Transitional cloacogenic carcinoma of the anal canal. Cancer. 1966;20:1727–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

