Geospatial immune variability illuminates differential evolution of lung adenocarcinoma
- PMID: 32461698
- PMCID: PMC7610840
- DOI: 10.1038/s41591-020-0900-x
Geospatial immune variability illuminates differential evolution of lung adenocarcinoma
Abstract
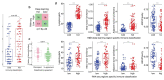
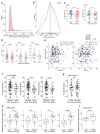
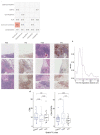
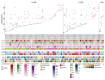
Remarkable progress in molecular analyses has improved our understanding of the evolution of cancer cells toward immune escape1-5. However, the spatial configurations of immune and stromal cells, which may shed light on the evolution of immune escape across tumor geographical locations, remain unaddressed. We integrated multiregion exome and RNA-sequencing (RNA-seq) data with spatial histology mapped by deep learning in 100 patients with non-small cell lung cancer from the TRACERx cohort6. Cancer subclones derived from immune cold regions were more closely related in mutation space, diversifying more recently than subclones from immune hot regions. In TRACERx and in an independent multisample cohort of 970 patients with lung adenocarcinoma, tumors with more than one immune cold region had a higher risk of relapse, independently of tumor size, stage and number of samples per patient. In lung adenocarcinoma, but not lung squamous cell carcinoma, geometrical irregularity and complexity of the cancer-stromal cell interface significantly increased in tumor regions without disruption of antigen presentation. Decreased lymphocyte accumulation in adjacent stroma was observed in tumors with low clonal neoantigen burden. Collectively, immune geospatial variability elucidates tumor ecological constraints that may shape the emergence of immune-evading subclones and aggressive clinical phenotypes.
Trial registration: ClinicalTrials.gov NCT01888601.
Conflict of interest statement
Y.Y. has received speakers bureau honoraria from Roche and is a consultant for Merck and Co Inc. C.S. receives grant support from Pfizer, AstraZeneca, BMS, Roche-Ventana, Boehringer-Ingelheim and Ono Pharmaceutical. C.S. has consulted for Pfizer, Novartis, GlaxoSmithKline, MSD, BMS, Celgene, AstraZeneca, Illumina, Genentech, Roche-Ventana, GRAIL, Medicxi, and the Sarah Cannon Research Institute. C.S. is a shareholder of Apogen Biotechnologies, Epic Bioscience, GRAIL, and has stock options in and is co-founder of Achilles Therapeutics. M.A.B. is a consultant for Achilles Therapeutics. S.L. receives research funding to her institution from Novartis, Bristol Meyers Squibb, Merck, Roche-Genentech, Puma Biotechnology, Pfizer, Eli Lilly and Seattle Genetics. S.L. has acted as consultant (not compensated) to Seattle Genetics, Pfizer, Novartis, BMS, Merck, AstraZeneca and RocheGenentech. S.L. has acted as consultant (paid to her institution) to Aduro Biotech, Novartis, and G1 Therapeutics. D.A.M. has received speaker’s fees from AstraZeneca. M.J.H. is a member of the Advisory Board for Achilles Therapeutics.
Figures






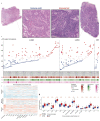
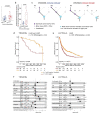
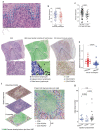
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U54 CA217376/CA/NCI NIH HHS/United States
- FC001202/WT_/Wellcome Trust/United Kingdom
- 21999/CRUK_/Cancer Research UK/United Kingdom
- C45982/A21808/CRUK_/Cancer Research UK/United Kingdom
- R01 CA185138/CA/NCI NIH HHS/United States
- 20466/CRUK_/Cancer Research UK/United Kingdom
- 30025/CRUK_/Cancer Research UK/United Kingdom
- FC001169/CRUK_/Cancer Research UK/United Kingdom
- 21808/CRUK_/Cancer Research UK/United Kingdom
- 211179/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- FC001169/WT_/Wellcome Trust/United Kingdom
- FC001202/CRUK_/Cancer Research UK/United Kingdom
- 24956/CRUK_/Cancer Research UK/United Kingdom
- 105104/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- MR/P014712/2/MRC_/Medical Research Council/United Kingdom
- MC_UP_1203/1/MRC_/Medical Research Council/United Kingdom
- 17786/CRUK_/Cancer Research UK/United Kingdom
- 25223/CRUK_/Cancer Research UK/United Kingdom
- MR/P014712/1/MRC_/Medical Research Council/United Kingdom
- C36463/A20764/CRUK_/Cancer Research UK/United Kingdom
- C11496/A17786/CRUK_/Cancer Research UK/United Kingdom
- C36463/A22246/CRUK_/Cancer Research UK/United Kingdom
- FC001169/MRC_/Medical Research Council/United Kingdom
- FC001202/MRC_/Medical Research Council/United Kingdom
- 22246/CRUK_/Cancer Research UK/United Kingdom
- 28706/CRUK_/Cancer Research UK/United Kingdom
- 29420/CRUK_/Cancer Research UK/United Kingdom
- 29569/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

