Synthesis, structure, photophysical and electrochemical properties of Ru(TFA)(CO)(PPh3)2(L) (L=2-phenylpyridine, 2- p-tolylpyridine) and Ru(CO)(PPhMe2)2(L)(L') (L= TFA, H) (L'= bipyridine, L'= 4,4'-dimethylbipyridine) relationships between ancillary ligand structure and luminescent properties
- PMID: 32461702
- PMCID: PMC7252952
- DOI: 10.1016/j.jorganchem.2017.03.026
Synthesis, structure, photophysical and electrochemical properties of Ru(TFA)(CO)(PPh3)2(L) (L=2-phenylpyridine, 2- p-tolylpyridine) and Ru(CO)(PPhMe2)2(L)(L') (L= TFA, H) (L'= bipyridine, L'= 4,4'-dimethylbipyridine) relationships between ancillary ligand structure and luminescent properties
Abstract
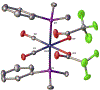
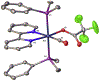
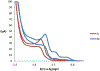
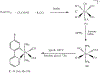
The synthesis, structure and photophysical properties of the complexes [Ru[(CO)(TFA) (PPh3)2(L)] [(L = ppy = 2-phenylpyridine, (1a); L = 2-(p-tolyl)pyridine] (1b), are reported. The complexes were characterized by UV-VIS, IR and NMR and by single-crystal X-ray diffraction techniques. We also report the synthesis, structure and photophysical properties of [Ru(CO)(L)(PPhMe2)2(L')]+[PF6]- [L' = bipyridine, L = TFA, (3a); L = H, (3b) and L = H, L' = 4,4'-dimethlyl bipyridine (3c)]. These compounds were characterized by UV-VIS, IR and NMR techniques and by a single crystal X-ray diffraction in the case of 3a. The solid state structure of [Ru(Me2PhP)2(CO)2(TFA)2 (2) which is the starting material for the synthesis 3a-3c is also reported to verify the trans relationship of the less bulky PPhMe2 and for comparison with the previously reported PPh3 analogs. The purpose of this study was to determine the impact, if any, of replacing bpy with ppy in the case of 1a and alkylation of the benzene ring in the case of 1b on the photophysical and electrochemical properties compared to related Ru(bpy) complexes. In contrast to the bpy analogs 1a and 1b showed reversible 1e- oxidations and blue-shifted MLCT absorptions. In the case of 3a-3c we were interested in the effect on the photophysical properties of substituting PPh3 with the less bulky but more electron donating PPhMe2. There were only minor changes in the photophysical and electrochemical properties relative to the previously reported PPh3 analogs.
Keywords: 2-Phenyl pyridine; Bipyridine; Electrochemistry; Phosphine ligands; Photophysical properties; Ru complexes.
Figures





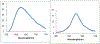
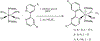
References
-
- Abbott G, The Development and Study of Surface Bound Ruthenium Complexes, Doctoral Dissertation, University of Montana, 2014.
-
- Alabbad S, Rosenberg E, Ross JBA, Ilu F submitted.
Grants and funding
LinkOut - more resources
Full Text Sources
