α 1-Adrenergic Receptor Blockade by Prazosin Synergistically Stabilizes Rat Peritoneal Mast Cells
- PMID: 32461978
- PMCID: PMC7243011
- DOI: 10.1155/2020/3214186
α 1-Adrenergic Receptor Blockade by Prazosin Synergistically Stabilizes Rat Peritoneal Mast Cells
Retraction in
-
Retracted: α1-Adrenergic Receptor Blockade by Prazosin Synergistically Stabilizes Rat Peritoneal Mast Cells.Biomed Res Int. 2024 Mar 20;2024:9759425. doi: 10.1155/2024/9759425. eCollection 2024. Biomed Res Int. 2024. PMID: 38550108 Free PMC article.
Abstract
Background: Adrenaline quickly inhibits the release of histamine from mast cells. Besides β 2-adrenergic receptors, several in vitro studies also indicate the involvement of α-adrenergic receptors in the process of exocytosis. Since exocytosis in mast cells can be detected electrophysiologically by the changes in the membrane capacitance (Cm), its continuous monitoring in the presence of drugs would determine their mast cell-stabilizing properties.
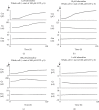
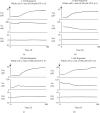
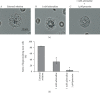
Methods: Employing the whole-cell patch-clamp technique in rat peritoneal mast cells, we examined the effects of adrenaline on the degranulation of mast cells and the increase in the Cm during exocytosis. We also examined the degranulation of mast cells in the presence or absence of α-adrenergic receptor agonists or antagonists.
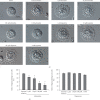
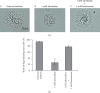
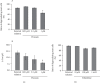
Results: Adrenaline dose-dependently suppressed the GTP-γ-S-induced increase in the Cm and inhibited the degranulation from mast cells, which was almost completely erased in the presence of butoxamine, a β 2-adrenergic receptor antagonist. Among α-adrenergic receptor agonists or antagonists, high-dose prazosin, a selective α 1-adrenergic receptor antagonist, significantly reduced the ratio of degranulating mast cells and suppressed the increase in the Cm. Additionally, prazosin augmented the inhibitory effects of adrenaline on the degranulation of mast cells.
Conclusions: This study provided electrophysiological evidence for the first time that adrenaline dose-dependently inhibited the process of exocytosis, confirming its usefulness as a potent mast cell stabilizer. The pharmacological blockade of α 1-adrenergic receptor by prazosin synergistically potentiated such mast cell-stabilizing property of adrenaline, which is primarily mediated by β 2-adrenergic receptors.
Copyright © 2020 Nozomu Abe et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sampson H. A., Muñoz-Furlong A., Campbell R. L., et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Journal of Allergy and Clinical Immunology. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

