Ceftolozane/Tazobactam for Treatment of Severe ESBL-Producing Enterobacterales Infections: A Multicenter Nationwide Clinical Experience (CEFTABUSE II Study)
- PMID: 32462046
- PMCID: PMC7237821
- DOI: 10.1093/ofid/ofaa139
Ceftolozane/Tazobactam for Treatment of Severe ESBL-Producing Enterobacterales Infections: A Multicenter Nationwide Clinical Experience (CEFTABUSE II Study)
Abstract
Background: Few data are reported in the literature about the outcome of patients with severe extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) infections treated with ceftolozane/tazobactam (C/T), in empiric or definitive therapy.
Methods: A multicenter retrospective study was performed in Italy (June 2016-June 2019). Successful clinical outcome was defined as complete resolution of clinical signs/symptoms related to ESBL-E infection and lack of microbiological evidence of infection. The primary end point was to identify predictors of clinical failure of C/T therapy.
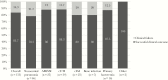
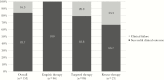
Results: C/T treatment was documented in 153 patients: pneumonia was the most common diagnosis (n = 46, 30%), followed by 34 cases of complicated urinary tract infections (22.2%). Septic shock was observed in 42 (27.5%) patients. C/T was used as empiric therapy in 46 (30%) patients and as monotherapy in 127 (83%) patients. Favorable clinical outcome was observed in 128 (83.7%) patients; 25 patients were considered to have failed C/T therapy. Overall, 30-day mortality was reported for 15 (9.8%) patients. At multivariate analysis, Charlson comorbidity index >4 (odds ratio [OR], 2.3; 95% confidence interval [CI], 1.9-3.5; P = .02), septic shock (OR, 6.2; 95% CI, 3.8-7.9; P < .001), and continuous renal replacement therapy (OR, 3.1; 95% CI, 1.9-5.3; P = .001) were independently associated with clinical failure, whereas empiric therapy displaying in vitro activity (OR, 0.12; 95% CI, 0.01-0.34; P < .001) and adequate source control of infection (OR, 0.42; 95% CI, 0.14-0.55; P < .001) were associated with clinical success.
Conclusions: Data show that C/T could be a valid option in empiric and/or targeted therapy in patients with severe infections caused by ESBL-producing Enterobacterales. Clinicians should be aware of the risk of clinical failure with standard-dose C/T therapy in septic patients receiving CRRT.
Keywords: CRRT; ESBL; Enterobacterales; ceftolozane/tazobactam; septic shock.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Rodríguez-Baño J, Navarro MD, Romero L, et al. Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Dis 2006; 43:1407–14. - PubMed
-
- Tumbarello M, Sanguinetti M, Montuori E, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 2007; 51:1987–94. - PMC - PubMed
-
- Russo A, Falcone M, Gutiérrez-Gutiérrez B, et al. ; REIPI/ESGBIS/INCREMENT investigators Predictors of outcome in patients with severe sepsis or septic shock due to extended-spectrum β-lactamase-producing Enterobacteriaceae. Int J Antimicrob Agents 2018; 52:577–85. - PubMed
-
- Pfaller MA, Bassetti M, Duncan LR, Castanheira M. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing urinary tract and intraabdominal infections in Europe: report from an antimicrobial surveillance programme (2012-15). J Antimicrob Chemother 2017; 72:1386–95. - PubMed

