Rapidly Switchable Universal CAR-T Cells for Treatment of CD123-Positive Leukemia
- PMID: 32462078
- PMCID: PMC7240059
- DOI: 10.1016/j.omto.2020.04.009
Rapidly Switchable Universal CAR-T Cells for Treatment of CD123-Positive Leukemia
Abstract
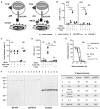
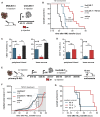
Chimeric antigen receptor T cells (CAR-T) targeting CD19 or B cell maturation antigen (BCMA) are highly effective against B cell malignancies. However, application of CAR-T to less differentially expressed targets remains a challenge due to lack of tumor-specific antigens and CAR-T controllability. CD123, a highly promising leukemia target, is expressed not only by leukemic and leukemia-initiating cells, but also by myeloid, hematopoietic progenitor, and certain endothelial cells. Thus, CAR-T lacking fine-tuned control mechanisms pose a high toxicity risk. To extend the CAR-T target landscape and widen the therapeutic window, we adapted our rapidly switchable universal CAR-T platform (UniCAR) to target CD123. UniCAR-T efficiently eradicated CD123+ leukemia in vitro and in vivo. Activation, cytolytic response, and cytokine release were strictly dependent on the presence of the CD123-specific targeting module (TM123) with comparable efficacy to CD123-specific CAR-T in vitro. We further demonstrated a pre-clinical proof of concept for the safety-switch mechanism using a hematotoxicity mouse model wherein TM123-redirected UniCAR-T showed reversible toxicity toward hematopoietic cells compared to CD123 CAR-T. In conclusion, UniCAR-T maintain full anti-leukemic efficacy, while ensuring rapid controllability to improve safety and versatility of CD123-directed immunotherapy. The safety and efficacy of UniCAR-T in combination with TM123 will now be assessed in a phase I clinical trial (ClinicalTrials.gov: NCT04230265).
Keywords: ALL; AML; CAR-T; CD123; UniCAR; adoptive cell therapy; immunotherapy.
© 2020 The Author(s).
Figures






References
-
- Ferrara J.L., Reddy P. Pathophysiology of graft-versus-host disease. Semin. Hematol. 2006;43:3–10. - PubMed
-
- Dykewicz C.A., Centers for Disease Control and Prevention (U.S.) Infectious Diseases Society of America. American Society of Blood and Marrow Transplantation Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 2001;33:139–144. - PubMed
-
- Coiffier B., Lepage E., Brière J., Herbrecht R., Tilly H., Bouabdallah R., Morel P., Van Den Neste E., Salles G., Gaulard P. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002;346:235–242. - PubMed
-
- Jin L., Lee E.M., Ramshaw H.S., Busfield S.J., Peoppl A.G., Wilkinson L., Guthridge M.A., Thomas D., Barry E.F., Boyd A. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

