CDK12-Altered Prostate Cancer: Clinical Features and Therapeutic Outcomes to Standard Systemic Therapies, Poly (ADP-Ribose) Polymerase Inhibitors, and PD-1 Inhibitors
- PMID: 32462107
- PMCID: PMC7252221
- DOI: 10.1200/po.19.00399
CDK12-Altered Prostate Cancer: Clinical Features and Therapeutic Outcomes to Standard Systemic Therapies, Poly (ADP-Ribose) Polymerase Inhibitors, and PD-1 Inhibitors
Abstract
Purpose: In prostate cancer, inactivating CDK12 mutations lead to gene fusion-induced neoantigens and possibly sensitivity to immunotherapy. We aimed to clinically, pathologically, and molecularly characterize CDK12-aberrant prostate cancers.
Methods: We conducted a retrospective multicenter study to identify patients with advanced prostate cancer who harbored somatic loss-of-function CDK12 mutations. We used descriptive statistics to characterize their clinical features and therapeutic outcomes (prostate-specific antigen [PSA] responses, progression-free survival [PFS]) to various systemic therapies, including sensitivity to poly (ADP-ribose) polymerase and PD-1 inhibitors.
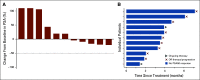
Results: Sixty men with at least monoallelic (51.7% biallelic) CDK12 alterations were identified across nine centers. Median age at diagnosis was 60.5 years; 71.7% and 28.3% were white and nonwhite, respectively; 93.3% had Gleason grade group 4-5; 15.4% had ductal/intraductal histology; 53.3% had metastases at diagnosis; and median PSA was 24.0 ng/mL. Of those who underwent primary androgen deprivation therapy for metastatic hormone-sensitive disease (n = 59), 79.7% had a PSA response, and median PFS was 12.3 months. Of those who received first-line abiraterone and enzalutamide for metastatic castration-resistant prostate cancer (mCRPC; n = 34), 41.2% had a PSA response, and median PFS was 5.3 months. Of those who received a first taxane chemotherapy for mCRPC (n = 22), 31.8% had a PSA response, and median PFS was 3.8 months. Eleven men received a PARP inhibitor (olaparib [n = 10], rucaparib [n = 1]), and none had a PSA response (median PFS, 3.6 months). Nine men received a PD-1 inhibitor as fourth- to sixth-line systemic therapy (pembrolizumab [n = 5], nivolumab [n = 4]); 33.3% had a PSA response, and median PFS was 5.4 months.
Conclusion: CDK12-altered prostate cancer is an aggressive subtype with poor outcomes to hormonal and taxane therapies as well as to PARP inhibitors. A proportion of these patients may respond favorably to PD-1 inhibitors, which implicates CDK12 deficiency in immunotherapy sensitivity.
Conflict of interest statement
Emmanuel S. Antonarakis
Pedro Isaacsson Velho
Neeraj Agarwal
Benjamin L. Maughan
Roberto Pili
Nabil Adra
Cora N. Sternberg
Panagiotis J. Vlachostergios
Scott T. Tagawa
Alan H. Bryce
Andrea L. McNatty
Zachery R. Reichert
Robert Dreicer
Oliver Sartor
Tamara L. Lotan
Maha Hussain
No other potential conflicts of interest were reported.
Figures

References
-
- Hussain M, Mateo J, Fizazi K, et al. PROfound: Phase 3 study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations. Ann Oncol. 2019;30:v881–v934.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

