Lipid droplets disrupt mechanosensing in human hepatocytes
- PMID: 32463334
- PMCID: PMC7468756
- DOI: 10.1152/ajpgi.00098.2020
Lipid droplets disrupt mechanosensing in human hepatocytes
Abstract
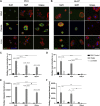
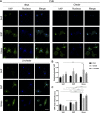
Hepatocellular carcinoma (HCC) is the fourth-leading cause of cancer death in the world. Although most cases occur in stiff, cirrhotic livers, and stiffness is a significant risk factor, HCC can also arise in noncirrhotic livers in the setting of nonalcoholic fatty liver disease (NAFLD). We hypothesized that lipid droplets in NAFLD might apply mechanical forces to the nucleus, functioning as mechanical stressors akin to stiffness. We investigated the effect of lipid droplets on cellular mechanosensing and found that primary human hepatocytes loaded with the fatty acids oleate and linoleate exhibited decreased stiffness-induced cell spreading and disrupted focal adhesions and stress fibers. The presence of large lipid droplets in hepatocytes resulted in increased nuclear localization of the mechano-sensor Yes-associated protein (YAP). In cirrhotic livers from patients with NAFLD, hepatocytes filled with large lipid droplets showed significantly higher nuclear localization of YAP as compared with cells with small lipid droplets. This work suggests that lipid droplets induce a mechanical signal that disrupts the ability of the hepatocyte to sense its underlying matrix stiffness and that the presence of lipid droplets can induce intracellular mechanical stresses.NEW & NOTEWORTHY This work examines the impact of lipid loading on mechanosensing by human hepatocytes. In cirrhotic livers, the presence of large (although not small) lipid droplets increased nuclear localization of the mechanotransducer YAP. In primary hepatocytes in culture, lipid droplets led to decreased stiffness-induced cell spreading and disrupted focal adhesions and stress fibers; the presence of large lipid droplets resulted in increased YAP nuclear localization. Collectively, the data suggest that lipid droplets induce intracellular mechanical stress.
Keywords: HCC; NAFLD; YAP; cirrhosis; lipid droplet.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






Similar articles
-
Lipid droplets are intracellular mechanical stressors that impair hepatocyte function.Proc Natl Acad Sci U S A. 2023 Apr 18;120(16):e2216811120. doi: 10.1073/pnas.2216811120. Epub 2023 Apr 10. Proc Natl Acad Sci U S A. 2023. PMID: 37036981 Free PMC article.
-
Deranged hepatocyte intracellular Ca2+ homeostasis and the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma.Cell Calcium. 2019 Sep;82:102057. doi: 10.1016/j.ceca.2019.102057. Epub 2019 Jul 26. Cell Calcium. 2019. PMID: 31401389 Review.
-
Biomechanics of cultured hepatic cells during different steatogenic hits.J Mech Behav Biomed Mater. 2019 Sep;97:296-305. doi: 10.1016/j.jmbbm.2019.05.036. Epub 2019 May 22. J Mech Behav Biomed Mater. 2019. PMID: 31151002
-
Nonalcoholic fatty liver disease promotes hepatocellular carcinoma through direct and indirect effects on hepatocytes.FEBS J. 2018 Feb;285(4):752-762. doi: 10.1111/febs.14209. Epub 2017 Sep 15. FEBS J. 2018. PMID: 28857485 Review.
-
Differences in metabolic and liver pathobiology induced by two dietary mouse models of nonalcoholic fatty liver disease.Am J Physiol Endocrinol Metab. 2020 Nov 1;319(5):E863-E876. doi: 10.1152/ajpendo.00321.2020. Epub 2020 Sep 14. Am J Physiol Endocrinol Metab. 2020. PMID: 32924526
Cited by
-
Methodological advancements in organ-specific ectopic lipid quantitative characterization: Effects of high fat diet on muscle and liver intracellular lipids.Mol Metab. 2023 Feb;68:101669. doi: 10.1016/j.molmet.2023.101669. Epub 2023 Jan 12. Mol Metab. 2023. PMID: 36642092 Free PMC article.
-
Lipophilic statins inhibit YAP coactivator transcriptional activity in HCC cells through Rho-mediated modulation of actin cytoskeleton.Am J Physiol Gastrointest Liver Physiol. 2023 Sep 1;325(3):G239-G250. doi: 10.1152/ajpgi.00089.2023. Epub 2023 Jun 27. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 37366601 Free PMC article.
-
Mechanobiology of portal hypertension.JHEP Rep. 2023 Aug 2;5(11):100869. doi: 10.1016/j.jhepr.2023.100869. eCollection 2023 Nov. JHEP Rep. 2023. PMID: 37841641 Free PMC article. Review.
-
Lipid droplets are intracellular mechanical stressors that impair hepatocyte function.Proc Natl Acad Sci U S A. 2023 Apr 18;120(16):e2216811120. doi: 10.1073/pnas.2216811120. Epub 2023 Apr 10. Proc Natl Acad Sci U S A. 2023. PMID: 37036981 Free PMC article.
-
Endomembranes: Unsung Heroes of Mechanobiology?Front Bioeng Biotechnol. 2020 Oct 22;8:597721. doi: 10.3389/fbioe.2020.597721. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195167 Free PMC article. Review.
References
-
- Adler M, Larocca L, Trovato FM, Marcinkowski H, Pasha Y, Taylor-Robinson SD. Evaluating the risk of hepatocellular carcinoma in patients with prominently elevated liver stiffness measurements by FibroScan: a multicentre study. HPB (Oxford) 18: 678–683, 2016. doi:10.1016/j.hpb.2016.05.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

