TUBB4A mutations result in both glial and neuronal degeneration in an H-ABC leukodystrophy mouse model
- PMID: 32463361
- PMCID: PMC7255805
- DOI: 10.7554/eLife.52986
TUBB4A mutations result in both glial and neuronal degeneration in an H-ABC leukodystrophy mouse model
Abstract
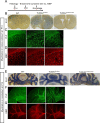
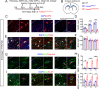
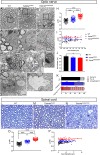
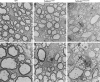
Mutations in TUBB4A result in a spectrum of leukodystrophy including Hypomyelination with Atrophy of Basal Ganglia and Cerebellum (H-ABC), a rare hypomyelinating leukodystrophy, often associated with a recurring variant p.Asp249Asn (D249N). We have developed a novel knock-in mouse model harboring heterozygous (Tubb4aD249N/+) and the homozygous (Tubb4aD249N/D249N) mutation that recapitulate the progressive motor dysfunction with tremor, dystonia and ataxia seen in H-ABC. Tubb4aD249N/D249N mice have myelination deficits along with dramatic decrease in mature oligodendrocytes and their progenitor cells. Additionally, a significant loss occurs in the cerebellar granular neurons and striatal neurons in Tubb4aD249N/D249N mice. In vitro studies show decreased survival and dysfunction in microtubule dynamics in neurons from Tubb4aD249N/D249N mice. Thus Tubb4aD249N/D249N mice demonstrate the complex cellular physiology of H-ABC, likely due to independent effects on oligodendrocytes, striatal neurons, and cerebellar granule cells in the context of altered microtubule dynamics, with profound neurodevelopmental deficits.
Keywords: TUBB4A; hypomyelination; leukodystrophy; microtubule; mouse; neurodegeneration; neuroscience.
Plain language summary
Inside human and other animal cells, filaments known as microtubules help support the shape of the cell and move proteins to where they need to be. Defects in microtubules may lead to disease. For example, genetic mutations affecting a microtubule component called TUBB4A cause a rare brain disease in humans known as H-ABC. Individuals with H-ABC display many symptoms including abnormal walking, speech defects, impaired swallowing, and several cognitive defects. Abnormalities in several areas of the brain, including the cerebellum and striatum contribute to these defects. . In these structures, the neurons that carry messages around the brain and their supporting cells, known as oligodendrocytes, die, which causes these parts of the brain to gradually waste away. At this time, there are no therapies available to treat H-ABC. Furthermore, research into the disease has been hampered by the lack of a suitable “model” in mice or other laboratory animals. To address this issue, Sase, Almad et al. generated mice carrying a mutation in a gene which codes for the mouse equivalent of the human protein TUBB4A. Experiments showed that the mutant mice had similar physical symptoms to humans with H-ABC, including an abnormal walking gait, poor coordination and involuntary movements such as twitching and reduced reflexes. H-ABC mice had smaller cerebellums than normal mice, which was consistent with the wasting away of the cerebellum observed in individuals with H-ABC. The mice also lost neurons in the striatum and cerebellum, and oligodendrocytes in the brain and spinal cord. Furthermore, the mutant TUBB4A protein affected the behavior and formation of microtubules in H-ABC mice. The findings of Sase, Almad et al. provide the first mouse model that shares many features of H-ABC disease in humans. This model provides a useful tool to study the disease and develop potential new therapies.
© 2020, Sase et al.
Conflict of interest statement
SS, AA, CB, PG, JL, AT, AP, TM, HP, DS, JC, JS, QP, EH, SS No competing interests declared, AV has research grants support by Gilead, Ionis, Eli Lilly, Illumina and Shire/Takeda, however none of these sources contributed to the current project.
Figures










References
-
- Baxi EG, DeBruin J, Jin J, Strasburger HJ, Smith MD, Orthmann-Murphy JL, Schott JT, Fairchild AN, Bergles DE, Calabresi PA. Lineage tracing reveals dynamic changes in oligodendrocyte precursor cells following cuprizone-induced demyelination. Glia. 2017;65:2087–2098. doi: 10.1002/glia.23229. - DOI - PMC - PubMed
-
- Biddle F, March E, Miller J. Research news. Mouse News Lett. 1973;48:26–28.
-
- Blumkin L, Halevy A, Ben-Ami-Raichman D, Dahari D, Haviv A, Sarit C, Lev D, van der Knaap MS, Lerman-Sagie T, Leshinsky-Silver E. Expansion of the spectrum of TUBB4A-related disorders: a new phenotype associated with a novel mutation in the TUBB4A gene. Neurogenetics. 2014;15:107–113. doi: 10.1007/s10048-014-0392-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

