True S-cones are concentrated in the ventral mouse retina and wired for color detection in the upper visual field
- PMID: 32463363
- PMCID: PMC7308094
- DOI: 10.7554/eLife.56840
True S-cones are concentrated in the ventral mouse retina and wired for color detection in the upper visual field
Abstract
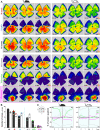
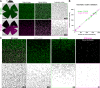
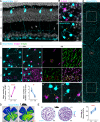
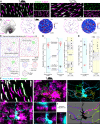
Color, an important visual cue for survival, is encoded by comparing signals from photoreceptors with different spectral sensitivities. The mouse retina expresses a short wavelength-sensitive and a middle/long wavelength-sensitive opsin (S- and M-opsin), forming opposing, overlapping gradients along the dorsal-ventral axis. Here, we analyzed the distribution of all cone types across the entire retina for two commonly used mouse strains. We found, unexpectedly, that 'true S-cones' (S-opsin only) are highly concentrated (up to 30% of cones) in ventral retina. Moreover, S-cone bipolar cells (SCBCs) are also skewed towards ventral retina, with wiring patterns matching the distribution of true S-cones. In addition, true S-cones in the ventral retina form clusters, which may augment synaptic input to SCBCs. Such a unique true S-cone and SCBC connecting pattern forms a basis for mouse color vision, likely reflecting evolutionary adaptation to enhance color coding for the upper visual field suitable for mice's habitat and behavior.
Keywords: blue bipolar cells; color vision; cone cluster; cone distribution; evolutionary biology; genuine s-cone; mammalian photoreceptor; mouse; neuroscience.
Plain language summary
Many primates, including humans, can see color better than most other mammals. This difference is due to the variety of light-detecting proteins – called opsins – that are produced in the eye by cells known as cones. While humans have three, mice only have two different opsins, known as S and M, which detect blue/UV and green light, respectively. Mouse cones produce either S-opsins, M-opsins or both. Fewer than 10 percent of cone cells in mice produce just the S-opsin, and these cells are essential for color vision. Mice are commonly used in scientific research, and so their vision has been well studied. However, previous research has produced conflicting results. Some studies report that cone cells that contain only S-opsin are evenly spread out across the retina. Other evidence suggests that color vision in mice exists only for the upper field of their vision, in other words, that mice can only distinguish colors that appeared above them. Nadal-Nicolás et al. set out to understand how to reconcile these contrasting findings. Molecular tools were used to detect S- and M-opsin in the retina of mice and revealed large differences between the lower part, known as the ventral retina, and the upper part, known as the dorsal retina. The ventral retina detects light coming from above the animal, and about a third of cone cells in this region produced exclusively S-opsin, compared to only 1 percent of cones in the dorsal retina. These S-opsin cone cells in the ventral retina group into clusters, where they connect with a special type of nerve cells that transmit this signal. To better understand these findings, Nadal-Nicolás et al. also studied albino mice. Although albino mice have a different distribution of S-opsin protein in the retina, the cone cells producing only S-opsin are similarly clustered in the ventral retina. This suggests that the concentration of S-opsin cone cells in the ventral retina is an important feature in mouse sight. This new finding corrects the misconception that S-opsin-only cone cells are evenly spread throughout the retina and supports the previous evidence that mouse color vision is greatest in the upper part of their field of vision. Nadal-Nicolás et al. suggest this arrangement could help the mice to detect predators that may attack them from above during the daytime. Together, these new findings could help to improve the design of future studies involving vision in mice and potentially other similar species.
Conflict of interest statement
FN, VK, JB, BP, AK, GZ, LD, WL No competing interests declared
Figures

References
-
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

