PET technology for drug development in psychiatry
- PMID: 32463584
- PMCID: PMC7722687
- DOI: 10.1002/npr2.12084
PET technology for drug development in psychiatry
Abstract
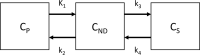
Positron emission tomography (PET) is a non-invasive imaging method to measure the molecule in vivo. PET imaging can evaluate the central nervous system drugs as target engagement in the human brain. For antipsychotic drugs, adequate dopamine D2 receptor occupancy ("therapeutic window") is reported to be from 65%-70% to 80% to achieve the antipsychotic effect without extrapyramidal symptoms. For antidepressants, the clinical threshold of serotonin transporter (5-HTT) occupancy is reported to be 70%-80% although the relation between the side effect and 5-HTT occupancy has not yet been established. Evaluation of norepinephrine transporter (NET) occupancy for antidepressant is ongoing as adequate PET radioligands for NET were developed recently. Measurement of the target occupancy has been a key element to evaluate the in vivo target engagement of the drugs. In order to evaluate new drug targets for disease conditions such as negative symptoms/cognitive impairment of schizophrenia and treatment-resistant depression, new PET radioligands need to be developed concurrently with the drug development.
Keywords: dopamine D2 receptor; norepinephrine transporter; occupancy; positron emission tomography; serotonin transporter.
© 2019 The Authors. Neuropsychopharmacology Reports published by John Wiley & Sons Australia, Ltd on behalf of the Japanese Society of NeuropsychoPharmacology.
Conflict of interest statement
AT is an employee of Takeda Pharmaceutical Company Limited. Other authors declare no conflict of interest.
Figures



References
-
- Halldin C, Gulyás B, Farde L. PET studies with carbon‐11 radioligands in neuropsychopharmacological drug development. Curr Pharm Des. 2001;7(18):1907–29. - PubMed
-
- Varnäs K, Varrone A, Farde L. Modeling of PET data in CNS drug discovery and development. J Pharmacokinet Pharmacodyn. 2013;40(3):267–79. - PubMed
-
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–9. - PubMed
-
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time‐activity measurements applied to [N‐11C‐methyl]‐(‐)‐cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10(5):740–7. - PubMed
-
- Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22(10):1271–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

