"Canopy Catalysts" for Alkyne Metathesis: Molybdenum Alkylidyne Complexes with a Tripodal Ligand Framework
- PMID: 32463684
- PMCID: PMC7322728
- DOI: 10.1021/jacs.0c04742
"Canopy Catalysts" for Alkyne Metathesis: Molybdenum Alkylidyne Complexes with a Tripodal Ligand Framework
Abstract
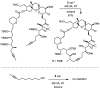
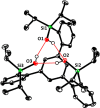
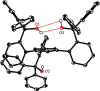
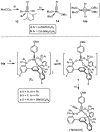
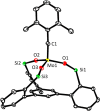
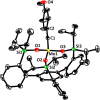
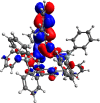
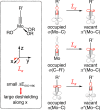
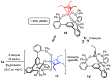
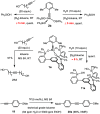
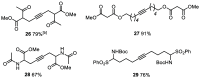
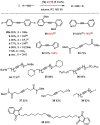
A new family of structurally well-defined molybdenum alkylidyne catalysts for alkyne metathesis, which is distinguished by a tripodal trisilanolate ligand architecture, is presented. Complexes of type 1 combine the virtues of previous generations of silanolate-based catalysts with a significantly improved functional group tolerance. They are easy to prepare on scale; the modularity of the ligand synthesis allows the steric and electronic properties to be fine-tuned and hence the application profile of the catalysts to be optimized. This opportunity is manifested in the development of catalyst 1f, which is as reactive as the best ancestors but exhibits an unrivaled scope. The new catalysts work well in the presence of unprotected alcohols and various other protic groups. The chelate effect entails even a certain stability toward water, which marks a big leap forward in metal alkylidyne chemistry in general. At the same time, they tolerate many donor sites, including basic nitrogen and numerous heterocycles. This aspect is substantiated by applications to polyfunctional (natural) products. A combined spectroscopic, crystallographic, and computational study provides insights into structure and electronic character of complexes of type 1. Particularly informative are a density functional theory (DFT)-based chemical shift tensor analysis of the alkylidyne carbon atom and 95Mo NMR spectroscopy; this analytical tool had been rarely used in organometallic chemistry before but turns out to be a sensitive probe that deserves more attention. The data show that the podand ligands render a Mo-alkylidyne a priori more electrophilic than analogous monodentate triarylsilanols; proper ligand tuning, however, allows the Lewis acidity as well as the steric demand about the central atom to be adjusted to the point that excellent performance of the catalyst is ensured.
Conflict of interest statement
The authors declare no competing financial interest.
Figures












Similar articles
-
Molybdenum Alkylidyne Complexes with Tripodal Silanolate Ligands: The Next Generation of Alkyne Metathesis Catalysts.Angew Chem Int Ed Engl. 2019 Oct 28;58(44):15690-15696. doi: 10.1002/anie.201908571. Epub 2019 Sep 17. Angew Chem Int Ed Engl. 2019. PMID: 31449713 Free PMC article.
-
From the Glovebox to the Benchtop: Air-Stable High Performance Molybdenum Alkylidyne Catalysts for Alkyne Metathesis.J Am Chem Soc. 2023 Dec 13;145(49):26993-27009. doi: 10.1021/jacs.3c10430. Epub 2023 Nov 30. J Am Chem Soc. 2023. PMID: 38032858 Free PMC article.
-
Canopy Catalysts for Alkyne Metathesis: Investigations into a Bimolecular Decomposition Pathway and the Stability of the Podand Cap.Chemistry. 2021 Oct 7;27(56):14025-14033. doi: 10.1002/chem.202102080. Epub 2021 Aug 26. Chemistry. 2021. PMID: 34293239 Free PMC article.
-
Organometallic Chemistry of Transition Metal Alkylidyne Complexes Centered at Metathesis Reactions.J Am Chem Soc. 2022 Jul 20;144(28):12546-12566. doi: 10.1021/jacs.2c01192. Epub 2022 Jul 6. J Am Chem Soc. 2022. PMID: 35793547 Review.
-
Well-Defined Alkyne Metathesis Catalysts: Developments and Recent Applications.Chemistry. 2019 Mar 1;25(13):3190-3208. doi: 10.1002/chem.201804511. Epub 2018 Dec 14. Chemistry. 2019. PMID: 30346054 Review.
Cited by
-
Impact of Ligands and Metals on the Formation of Metallacyclic Intermediates and a Nontraditional Mechanism for Group VI Alkyne Metathesis Catalysts.J Am Chem Soc. 2021 Jun 23;143(24):9026-9039. doi: 10.1021/jacs.1c01843. Epub 2021 Jun 10. J Am Chem Soc. 2021. PMID: 34110130 Free PMC article.
-
Productive Alkyne Metathesis with "Canopy Catalysts" Mandates Pseudorotation.J Am Chem Soc. 2021 Apr 21;143(15):5643-5648. doi: 10.1021/jacs.1c01404. Epub 2021 Apr 7. J Am Chem Soc. 2021. PMID: 33826335 Free PMC article.
-
By-design molecular architectures via alkyne metathesis.Chem Sci. 2021 May 22;12(28):9591-9606. doi: 10.1039/d1sc01881g. eCollection 2021 Jul 21. Chem Sci. 2021. PMID: 34349932 Free PMC article. Review.
-
Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets.Angew Chem Int Ed Engl. 2021 Mar 1;60(10):5316-5322. doi: 10.1002/anie.202015243. Epub 2021 Jan 28. Angew Chem Int Ed Engl. 2021. PMID: 33289954 Free PMC article.
-
183 W NMR Spectroscopy Guides the Search for Tungsten Alkylidyne Catalysts for Alkyne Metathesis.Angew Chem Int Ed Engl. 2020 Nov 23;59(48):21758-21768. doi: 10.1002/anie.202009975. Epub 2020 Sep 24. Angew Chem Int Ed Engl. 2020. PMID: 32820864 Free PMC article.
References
-
- Thompson R. R.; Rotella M. E.; Du P.; Zhou X.; Fronczek F. R.; Kumar R.; Gutierrez O.; Lee S. Siloxide Podand Ligand Scaffold for Molybdenum-Catalyzed Alkyne Metathesis and Isolation of a Dynamic Metallatetrahedrane Intermediate. Organometallics 2019, 38, 4054–4059. 10.1021/acs.organomet.9b00430. - DOI
-
- Heppekausen J.; Stade R.; Goddard R.; Fürstner A. Practical New Silyloxy-based Alkyne Metathesis Catalysts with Optimized Activity and Selectivity Profiles. J. Am. Chem. Soc. 2010, 132, 11045–11057. 10.1021/ja104800w. - DOI - PubMed
-
For the lead finding that siloxides are outstanding ligands for Mo-based catalysts, see:
- Bindl M.; Stade R.; Heilmann E. K.; Picot A.; Goddard R.; Fürstner A. Molybdenum Nitride Complexes with Ph3SiO- Ligands are Exceedingly Practical and Tolerant Precatalysts for Alkyne Metathesis and Efficient Nitrogen Transfer Agents. J. Am. Chem. Soc. 2009, 131, 9468–9470. 10.1021/ja903259g. - DOI - PubMed
-
- Persich P.; Llaveria J.; Lhermet R.; de Haro T.; Stade R.; Kondoh A.; Fürstner A. Increasing the Structural Span of Alkyne Metathesis. Chem. - Eur. J. 2013, 19, 13047–13058. 10.1002/chem.201302320. - DOI - PubMed
- Lhermet R.; Fürstner A. Cross-Metathesis of Terminal Alkynes. Chem. - Eur. J. 2014, 20, 13188–13193. 10.1002/chem.201404166. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

