An osteocalcin-deficient mouse strain without endocrine abnormalities
- PMID: 32463812
- PMCID: PMC7255615
- DOI: 10.1371/journal.pgen.1008361
An osteocalcin-deficient mouse strain without endocrine abnormalities
Abstract
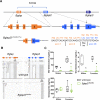
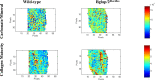
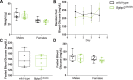
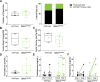
Osteocalcin (OCN), the most abundant noncollagenous protein in the bone matrix, is reported to be a bone-derived endocrine hormone with wide-ranging effects on many aspects of physiology, including glucose metabolism and male fertility. Many of these observations were made using an OCN-deficient mouse allele (Osc-) in which the 2 OCN-encoding genes in mice, Bglap and Bglap2, were deleted in ES cells by homologous recombination. Here we describe mice with a new Bglap and Bglap2 double-knockout (dko) allele (Bglap/2p.Pro25fs17Ter) that was generated by CRISPR/Cas9-mediated gene editing. Mice homozygous for this new allele do not express full-length Bglap or Bglap2 mRNA and have no immunodetectable OCN in their serum. FTIR imaging of cortical bone in these homozygous knockout animals finds alterations in the collagen maturity and carbonate to phosphate ratio in the cortical bone, compared with wild-type littermates. However, μCT and 3-point bending tests do not find differences from wild-type littermates with respect to bone mass and strength. In contrast to the previously reported OCN-deficient mice with the Osc-allele, serum glucose levels and male fertility in the OCN-deficient mice with the Bglap/2pPro25fs17Ter allele did not have significant differences from wild-type littermates. We cannot explain the absence of endocrine effects in mice with this new knockout allele. Possible explanations include the effects of each mutated allele on the transcription of neighboring genes, or differences in genetic background and environment. So that our findings can be confirmed and extended by other interested investigators, we are donating this new Bglap and Bglap2 double-knockout strain to the Jackson Laboratories for academic distribution.
Conflict of interest statement
BOW is the recipient of a sponsored research agreement from Janssen Pharmaceuticals for work not directly related to these studies. BOW also is a member of the Scientific Advisory Board for Surrozen.
Figures

Comment in
-
Osteocalcin promotes bone mineralization but is not a hormone.PLoS Genet. 2020 Jun 2;16(6):e1008714. doi: 10.1371/journal.pgen.1008714. eCollection 2020 Jun. PLoS Genet. 2020. PMID: 32484816 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

