The haemodynamics of the human placenta in utero
- PMID: 32463837
- PMCID: PMC7255609
- DOI: 10.1371/journal.pbio.3000676
The haemodynamics of the human placenta in utero
Abstract
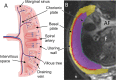
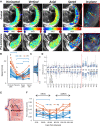
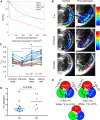
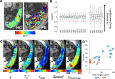
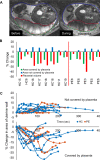
We have used magnetic resonance imaging (MRI) to provide important new insights into the function of the human placenta in utero. We have measured slow net flow and high net oxygenation in the placenta in vivo, which are consistent with efficient delivery of oxygen from mother to fetus. Our experimental evidence substantiates previous hypotheses on the effects of spiral artery remodelling in utero and also indicates rapid venous drainage from the placenta, which is important because this outflow has been largely neglected in the past. Furthermore, beyond Braxton Hicks contractions, which involve the entire uterus, we have identified a new physiological phenomenon, the 'utero-placental pump', by which the placenta and underlying uterine wall contract independently of the rest of the uterus, expelling maternal blood from the intervillous space.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Trophoblast plugs: impact on utero-placental haemodynamics and spiral artery remodelling.Hum Reprod. 2018 Aug 1;33(8):1430-1441. doi: 10.1093/humrep/dey225. Hum Reprod. 2018. PMID: 29955830
-
The effects of maternal position, in late gestation pregnancy, on placental blood flow and oxygenation: an MRI study.J Physiol. 2021 Mar;599(6):1901-1915. doi: 10.1113/JP280569. Epub 2021 Jan 18. J Physiol. 2021. PMID: 33369732 Free PMC article.
-
First trimester uterine, placental and yolk sac haemodynamics in pre-eclampsia and preterm labour.Hum Reprod. 2004 Mar;19(3):729-33. doi: 10.1093/humrep/deh106. Epub 2004 Jan 29. Hum Reprod. 2004. PMID: 14998978
-
Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology.Hum Reprod Update. 2014 Mar-Apr;20(2):293-307. doi: 10.1093/humupd/dmt054. Epub 2013 Oct 16. Hum Reprod Update. 2014. PMID: 24132226 Review.
-
Pathophysiology of placental-derived fetal growth restriction.Am J Obstet Gynecol. 2018 Feb;218(2S):S745-S761. doi: 10.1016/j.ajog.2017.11.577. Am J Obstet Gynecol. 2018. PMID: 29422210 Review.
Cited by
-
Placenta in the Critically Ill Mother.Indian J Crit Care Med. 2021 Dec;25(Suppl 3):S200-S205. doi: 10.5005/jp-journals-10071-24024. Indian J Crit Care Med. 2021. PMID: 35615604 Free PMC article.
-
Assessing within-subject rates of change of placental MRI diffusion metrics in normal pregnancy.Magn Reson Med. 2023 Sep;90(3):1137-1150. doi: 10.1002/mrm.29665. Epub 2023 May 15. Magn Reson Med. 2023. PMID: 37183839 Free PMC article.
-
A massively multi-scale approach to characterizing tissue architecture by synchrotron micro-CT applied to the human placenta.J R Soc Interface. 2021 Jun;18(179):20210140. doi: 10.1098/rsif.2021.0140. Epub 2021 Jun 2. J R Soc Interface. 2021. PMID: 34062108 Free PMC article.
-
Compromised barrier integrity of human feto-placental vessels from gestational diabetic pregnancies is related to downregulation of occludin expression.Diabetologia. 2021 Jan;64(1):195-210. doi: 10.1007/s00125-020-05290-6. Epub 2020 Oct 1. Diabetologia. 2021. PMID: 33001231 Free PMC article.
-
The human placenta project: Funded studies, imaging technologies, and future directions.Placenta. 2023 Oct;142:27-35. doi: 10.1016/j.placenta.2023.08.067. Epub 2023 Aug 21. Placenta. 2023. PMID: 37634371 Free PMC article. Review.
References
-
- Peter Kaufmann. Comparative Placentation: Guinea Pig [Internet]. UCSF website. 2004 [cited 2019 Jan 12]. Available from: http://placentation.ucsd.edu/guinea.htm.
-
- McNanley T, Woods J. Placental Physiology [Internet]. Glob Libr Women’s Med. 2008. [cited 2020 May 11]. Available from: http://www.glowm.com/index.html?p=glowm.cml/section_view&articleid=195.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

