The role of nitric oxide in brain disorders: Autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders
- PMID: 32464501
- PMCID: PMC7256645
- DOI: 10.1016/j.redox.2020.101567
The role of nitric oxide in brain disorders: Autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders
Abstract
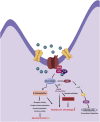
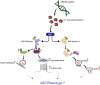
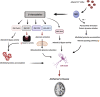
Nitric oxide (NO) is a multifunctional signalling molecule and a neurotransmitter that plays an important role in physiological and pathophysiological processes. In physiological conditions, NO regulates cell survival, differentiation and proliferation of neurons. It also regulates synaptic activity, plasticity and vesicle trafficking. NO affects cellular signalling through protein S-nitrosylation, the NO-mediated posttranslational modification of cysteine thiols (SNO). SNO can affect protein activity, protein-protein interaction and protein localization. Numerous studies have shown that excessive NO and SNO can lead to nitrosative stress in the nervous system, contributing to neuropathology. In this review, we summarize the role of NO and SNO in the progression of neurodevelopmental, psychiatric and neurodegenerative disorders, with special attention to autism spectrum disorder (ASD). We provide mechanistic insights into the contribution of NO in diverse brain disorders. Finally, we suggest that pharmacological agents that can inhibit or augment the production of NO as well as new approaches to modulate the formation of SNO-proteins can serve as a promising approach for the treatment of diverse brain disorders.
Keywords: Alzheimer's disease; Autism spectrum disorder; Brain disorders; Neurodegeneration; Neurodevelopmental disorders; Nitric oxide; Psychiatry; S-nitrosylation; SHANK3.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no competing interests to declare.
Figures

References
-
- Bredt D.S., Snyder S.H. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. - PubMed
-
- Bredt D.S., Hwang P.M. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351(6329):714. - PubMed
-
- Förstermann U. Isoforms of nitric oxide synthase characterization and purification from different cell types. Biochem. Pharmacol. 1991;42(10):1849–1857. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

