Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study
- PMID: 32464528
- PMCID: PMC7251365
- DOI: 10.1016/j.ebiom.2020.102785
Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study
Abstract
Background: Serum biomarkers may inform and improve care in traumatic brain injury (TBI). We aimed to correlate serum biomarkers with clinical severity, care path and imaging abnormalities in TBI, and explore their incremental value over clinical characteristics in predicting computed tomographic (CT) abnormalities.
Methods: We analyzed six serum biomarkers (S100B, NSE, GFAP, UCH-L1, NFL and t-tau) obtained <24 h post-injury from 2867 patients with any severity of TBI in the Collaborative European NeuroTrauma Effectiveness Research (CENTER-TBI) Core Study, a prospective, multicenter, cohort study. Univariable and multivariable logistic regression analyses were performed. Discrimination was assessed by the area under the receiver operating characteristic curve (AUC) with 95% confidence intervals.
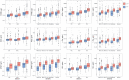
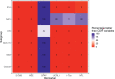
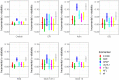
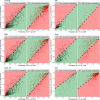
Findings: All biomarkers scaled with clinical severity and care path (ER only, ward admission, or ICU), and with presence of CT abnormalities. GFAP achieved the highest discrimination for predicting CT abnormalities (AUC 0•89 [95%CI: 0•87-0•90]), with a 99% likelihood of better discriminating CT-positive patients than clinical characteristics used in contemporary decision rules. In patients with mild TBI, GFAP also showed incremental diagnostic value: discrimination increased from 0•84 [95%CI: 0•83-0•86] to 0•89 [95%CI: 0•87-0•90] when GFAP was included. Results were consistent across strata, and injury severity. Combinations of biomarkers did not improve discrimination compared to GFAP alone.
Interpretation: Currently available biomarkers reflect injury severity, and serum GFAP, measured within 24 h after injury, outperforms clinical characteristics in predicting CT abnormalities. Our results support the further development of serum GFAP assays towards implementation in clinical practice, for which robust clinical assay platforms are required.
Funding: CENTER-TBI study was supported by the European Union 7th Framework program (EC grant 602150).
Keywords: Biomarkers; Clinical decision rule; Computerized tomography; Diagnostic; GFAP; Injury severity; Serum; Traumatic brain injury.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors declare support to the CENTER-TBI project by funding bodies and other organizations as listed in the acknowledgement section. DKM reports grants from National Institute for Health Research (NIHR; UK), during the conduct of the study; grants, personal fees and non-financial support from GlaxoSmithKline, personal fees from Neurotrauma Sciences, personal fees from Lantmaanen AB, personal fees from Pressura, personal fees from Pfizer, outside the submitted work. AIRM declares consulting fees from PresSura Neuro, Integra Life Sciences and NeuroTrauma Sciences. EC, KA and AB report grants Higher Education Institutional Excellence Programme – Grant No. 20765-3/2018/FEKUTSTRAT, FIKP II/S, EFOP-3.6.2.-16-2017-00008, GINOP-2.3.2-15-2016-00048, and GINOP-2.3.3-15-2016-00032 and the Hungarian Brain Research Program 2.0 Grant No. 2017-1.2.1-NKP-2017-00002. KKWW is co-founder and shareholder of Gryphon Bio. and was co-founder and shareholder of Banyan Biomarkers. VFJN is supported by an Academy of Medical Sciences/The Health Foundation Clinician Scientist Fellowship. SR reports funding from the Wellcome Trust for a Clinician Ph.D. Fellowship. BYG, FL, SM, EWS, JV, THvdV, HX, and ZY declare no competing interests.
Figures

Comment in
-
Searching for a traumatic brain injury biomarker to aid clinical decision making in the emergency department.EBioMedicine. 2020 Jun;56:102798. doi: 10.1016/j.ebiom.2020.102798. Epub 2020 May 5. EBioMedicine. 2020. PMID: 32502963 Free PMC article. No abstract available.
References
-
- Maas A.I.R., Menon D.K., Adelson P.D. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. - PubMed
-
- Yue J.K., Yuh E.L., Korley F.K. Association between plasma GFAP concentrations and MRI abnormalities in patients with CT-negative traumatic brain injury in the TRACK-TBI cohort: a prospective multicentre study. Lancet Neurol. 2019;18(10):953–961. - PubMed
-
- Stiell I.G., Wells G.A., Vandemheen K. The Canadian CT head rule for patients with minor head injury. Lancet. 2001;357(9266):1391–1396. - PubMed
-
- National_Clinical_Guideline_C._National_Clinical_Guidance_Centre. CG 176 . Vol. 2014. National Institute for Health and Care Excellence; 2014. (Head injury: triage, assessment, investigation and early management of head injury in children, young people and adults). - PubMed

