Insights into Manipulating Postprandial Energy Expenditure to Manage Weight Gain in Polycystic Ovary Syndrome
- PMID: 32464593
- PMCID: PMC7256642
- DOI: 10.1016/j.isci.2020.101164
Insights into Manipulating Postprandial Energy Expenditure to Manage Weight Gain in Polycystic Ovary Syndrome
Abstract
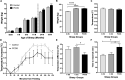
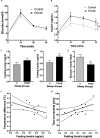
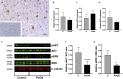
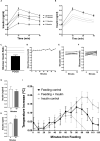
Women with polycystic ovary syndrome (PCOS) are more likely to be obese and have difficulty in losing weight. They demonstrate an obesity-independent deficit in adaptive energy expenditure. We used a clinically realistic preclinical model to investigate the molecular basis for the reduced postprandial thermogenesis (PPT) and develop a therapeutic strategy to normalize this deficit. Sheep exposed to increased androgens before birth develop the clinical features of PCOS. In adulthood they develop obesity and demonstrate an obesity-independent reduction in PPT. This is associated with reduced adipose tissue uncoupling protein expression and adipose tissue noradrenaline concentrations. These sheep are insulin resistant with reduced insulin signaling in the brain. Increasing brain insulin concentrations using intranasal insulin administration increased PPT in PCOS sheep without any effects on blood glucose concentrations. Intranasal insulin administration with food is a potential novel strategy to improve adaptive energy expenditure and normalize the responses to weight loss strategies in women with PCOS.
Keywords: Biological Sciences; Pathophysiology; Sheep Physiology.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of Interests The authors declare no competing interests.
Figures


References
-
- Altieri P., Cavazza C., Pasqui F., Morselli A.M., Gambineri A., Pasquali R. Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin. Endocrinol. 2013;78:52–59. - PubMed
-
- Álvarez-Blasco F., Luque-Ramírez M., Escobar-Morreale H.F. Diet composition and physical activity in overweight and obese premenopausal women with or without polycystic ovary syndrome. Gynecol. Endocrinol. 2011;27:978–981. - PubMed
-
- Astrup A., Simonsen L., Bülow J., Madsen J., Christensen N.J. Epinephrine mediates facultative carbohydrate-induced thermogenesis in human skeletal muscle. Am. J. Physiol. 1989;257:E340–E345. - PubMed
-
- Barnes R.B., Rosenfield R.L., Ehrmann D.A., Cara J.F., Cuttler L., Levitsky L.L., Rosenthal I.M. Ovarian hyperandrogenism as a result of congenital adrenal virilising disorders: evidence for prenatal masculinisation of neuroendocrine function in women. J. Clin. Endocrinol. Metab. 1994;79:1328–1333. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

