Platelet Transforming Growth Factor-β1 Induces Liver Sinusoidal Endothelial Cells to Secrete Interleukin-6
- PMID: 32466100
- PMCID: PMC7290849
- DOI: 10.3390/cells9051311
Platelet Transforming Growth Factor-β1 Induces Liver Sinusoidal Endothelial Cells to Secrete Interleukin-6
Abstract
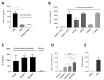
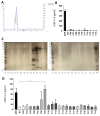
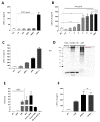
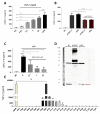
The roles and interactions of platelets and liver sinusoidal endothelial cells in liver regeneration are unclear, and the trigger that initiates hepatocyte proliferation is unknown. We aimed to identify the key factors released by activated platelets that induce liver sinusoidal endothelial cells to produce interleukin-6 (IL-6), a cytokine implicated in the early phase of liver regeneration. We characterized the releasate of activated platelets inducing the in vitro production of IL-6 by mouse liver sinusoidal endothelial cells and observed that the stimulating factor was a thermolabile protein. Following gel filtration, a single fraction of activated platelet releasate induced a maximal IL-6 secretion by liver sinusoidal endothelial cells (90.2 ± 13.9 versus control with buffer, 9.0 ± 0.8 pg/mL, p < 0.05). Mass spectroscopy analysis of this fraction, followed by in silico processing, resulted in a reduced list of 18 candidates. Several proteins from the list were tested, and only recombinant transforming growth factor β1 (TGF-β1) resulted in an increased IL-6 production up to 242.7 ± 30.5 pg/mL, which was comparable to non-fractionated platelet releasate effect. Using neutralizing anti-TGF-β1 antibody or a TGF-β1 receptor inhibitor, IL-6 production by liver sinusoidal endothelial cells was dramatically reduced. These results support a role of platelet TGF-β1 β1 in the priming phase of liver regeneration.
Keywords: Interleukin-6; hepatocytes; liver regeneration; livers sinusoidal cells; transforming growth factor β; von Willebrand factor.
Conflict of interest statement
S.B and A.T. are working for Regen Lab SA.
Figures


References
-
- Meyer J., Balaphas A., Fontana P., Sadoul K., Morel P., Gonelle-Gispert C., Bühler L. Platelets in liver regeneration. ISBT Sci. Ser. 2017;12:455–462. doi: 10.1111/voxs.12382. - DOI
-
- Peng Q., Yeh H., Wei L., Enjyoj K., Machaidze Z., Csizmad E., Schuetz C., Lee K.M., Deng S., Robson S.C., et al. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS ONE. 2012;7:e47273. doi: 10.1371/journal.pone.0047273. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

