Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics
- PMID: 32466129
- PMCID: PMC7290391
- DOI: 10.3390/cells9051313
Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics
Abstract
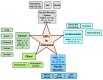
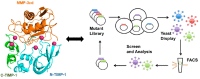
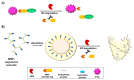
The metalloproteinase (MP) family of zinc-dependent proteases, including matrix metalloproteinases (MMPs), a disintegrin and metalloproteases (ADAMs), and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) plays a crucial role in the extracellular matrix (ECM) remodeling and degradation activities. A wide range of substrates of the MP family includes ECM components, chemokines, cell receptors, and growth factors. Metalloproteinases activities are tightly regulated by proteolytic activation and inhibition via their natural inhibitors, tissue inhibitors of metalloproteinases (TIMPs), and the imbalance of the activation and inhibition is responsible in progression or inhibition of several diseases, e.g., cancer, neurological disorders, and cardiovascular diseases. We provide an overview of the structure, function, and the multifaceted role of MMPs, ADAMs, and TIMPs in several diseases via their cellular functions such as proteolysis of other cell signaling factors, degradation and remodeling of the ECM, and other essential protease-independent interactions in the ECM. The significance of MP inhibitors targeting specific MMP or ADAMs with high selectivity is also discussed. Recent advances and techniques used in developing novel MP inhibitors and MP responsive drug delivery tools are also reviewed.
Keywords: ADAMs; MMP inhibitors; MMP-responsive therapeutics; MMPs; TIMPs; a disintegrin and metalloproteases; matrix metalloproteinases; metalloproteinases; metzincins; tissue inhibitors of metalloproteinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

