Acute Induction of Translocon-Mediated Ca2+ Leak Protects Cardiomyocytes Against Ischemia/Reperfusion Injury
- PMID: 32466308
- PMCID: PMC7290748
- DOI: 10.3390/cells9051319
Acute Induction of Translocon-Mediated Ca2+ Leak Protects Cardiomyocytes Against Ischemia/Reperfusion Injury
Abstract
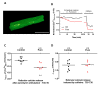
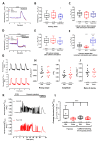
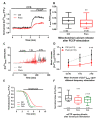
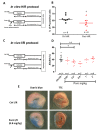
During myocardial infarction, dysregulation of Ca2+ homeostasis between the reticulum, mitochondria, and cytosol occurs in cardiomyocytes and leads to cell death. Ca2+ leak channels are thought to be key regulators of the reticular Ca2+ homeostasis and cell survival. The present study aimed to determine whether a particular reticular Ca2+ leak channel, the translocon, also known as translocation channel, could be a relevant target against ischemia/reperfusion-mediated heart injury. To achieve this objective, we first used an intramyocardial adenoviral strategy to express biosensors in order to assess Ca2+ variations in freshly isolated adult mouse cardiomyocytes to show that translocon is a functional reticular Ca2+ leak channel. Interestingly, translocon activation by puromycin mobilized a ryanodine receptor (RyR)-independent reticular Ca2+ pool and did not affect the excitation-concentration coupling. Second, puromycin pretreatment decreased mitochondrial Ca2+ content and slowed down the mitochondrial permeability transition pore (mPTP) opening and the rate of cytosolic Ca2+ increase during hypoxia. Finally, this translocon pre-activation also protected cardiomyocytes after in vitro hypoxia reoxygenation and reduced infarct size in mice submitted to in vivo ischemia-reperfusion. Altogether, our report emphasizes the role of translocon in cardioprotection and highlights a new paradigm in cardioprotection by functionally uncoupling the RyR-dependent Ca2+ stores and translocon-dependent Ca2+ stores.
Keywords: Ca2+ leak channel; cardioprotection; ischemia-reperfusion; reticulum; translocon.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

