DFT Prediction of Factors Affecting the Structural Characteristics, the Transition Temperature and the Electronic Density of Some New Conjugated Polymers
- PMID: 32466422
- PMCID: PMC7361686
- DOI: 10.3390/polym12061207
DFT Prediction of Factors Affecting the Structural Characteristics, the Transition Temperature and the Electronic Density of Some New Conjugated Polymers
Abstract
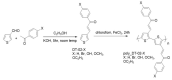
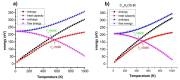
Conjugated polymers are promising materials for various cutting-edge technologies, especially for organic conducting materials and in the energy field. In this work, we have synthesized a new conjugated polymer and investigated the effect of distance between bond layers, side-chain functional groups (H, Br, OH, OCH3 and OC2H5) on structural characteristics, phase transition temperature (T), and electrical structure of C13H8OS using Density Functional Theory (DFT). The structural characteristics were determined by the shape, network constant (a, b and c), bond length (C-C, C-H, C-O, C-S, C-Br and O-H), phase transition temperatures, and the total energy (Etot) on a base cell. Our finding shows that the increase of layer thickness (h) of C13H8OS-H has a negligible effect on the transition temperature, while the energy bandgap (Eg) increases from 1.646 eV to 1.675 eV. The calculation of bond length with different side chain groups was carried out for which C13H8OS-H has C-H = 1.09 Å; C13H8OS-Br has C-Br = 1.93 Å; C13H8OS-OH has C-O = 1.36 Å, O-H = 0.78 Å; C13H8OS-OCH3 has C-O = 1.44 Å, O-H =1.10 Å; C13H8OS-OC2H5 has C-O = 1.45 Å, C-C = 1.51Å, C-H = 1.10 Å. The transition temperature (T) for C13H8OS-H was 500 K < T < 562 K; C13H8OS-Br was 442 K < T < 512 K; C13H8OS-OH was 487 K < T < 543 K; C13H8OS-OCH3 was 492 K < T < 558 K; and C13H8OS-OC2H5 was 492 K < T < 572 K. The energy bandgap (Eg) of Br is of Eg = 1.621 eV, the doping of side chain groups H, OH, OCH3, and OC2H5, leads to an increase of Eg from 1.621 eV to 1.646, 1.697, 1.920, and 2.04 eV, respectively.
Keywords: bond length; conjugated polymers; density functional theory; transition temperatures.
Conflict of interest statement
The authors declare no conflict of interest; The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results
Figures




Similar articles
-
Effect of doped H, Br, Cu, Kr, Ge, As and Fe on structural features and bandgap of poly C13H8OS-X: a DFT calculation.Des Monomers Polym. 2021 Feb 2;24(1):53-62. doi: 10.1080/15685551.2021.1877431. Des Monomers Polym. 2021. PMID: 33658884 Free PMC article.
-
DFT study on some polythiophenes containing benzo[d]thiazole and benzo[d]oxazole: structure and band gap.Des Monomers Polym. 2021 Sep 6;24(1):274-284. doi: 10.1080/15685551.2021.1971376. eCollection 2021. Des Monomers Polym. 2021. PMID: 34512118 Free PMC article.
-
First-Principles Study on the Half-Metallicity of New MXene Materials Nd2NT2 (T = OH, O, S, F, Cl, and Br).Front Chem. 2022 Feb 10;9:832449. doi: 10.3389/fchem.2021.832449. eCollection 2021. Front Chem. 2022. PMID: 35223780 Free PMC article.
-
Importance of halogen···halogen contacts for the structural and magnetic properties of CuX2(pyrazine-N,N′-dioxide)(H2O)2 (X = Cl and Br).Inorg Chem. 2012 Feb 20;51(4):2121-9. doi: 10.1021/ic201924q. Epub 2012 Feb 1. Inorg Chem. 2012. PMID: 22296451
-
Synthesis of Conjugated Polymers via Transition Metal Catalysed C-H Bond Activation.Chem Asian J. 2021 Oct 4;16(19):2896-2919. doi: 10.1002/asia.202100749. Epub 2021 Sep 3. Chem Asian J. 2021. PMID: 34390547 Review.
Cited by
-
Effect of doped H, Br, Cu, Kr, Ge, As and Fe on structural features and bandgap of poly C13H8OS-X: a DFT calculation.Des Monomers Polym. 2021 Feb 2;24(1):53-62. doi: 10.1080/15685551.2021.1877431. Des Monomers Polym. 2021. PMID: 33658884 Free PMC article.
-
DFT study on some polythiophenes containing benzo[d]thiazole and benzo[d]oxazole: structure and band gap.Des Monomers Polym. 2021 Sep 6;24(1):274-284. doi: 10.1080/15685551.2021.1971376. eCollection 2021. Des Monomers Polym. 2021. PMID: 34512118 Free PMC article.
-
Synthesis and characterization of some novel polythiophene derivatives containing pyrazoline.Des Monomers Polym. 2022 Jun 9;25(1):136-147. doi: 10.1080/15685551.2022.2086413. eCollection 2022. Des Monomers Polym. 2022. PMID: 35693727 Free PMC article.
-
Crystal structure of potassium hydrogen bis-((E)-2-{4-[3-(thio-phen-3-yl)acrylo-yl]phen-oxy}acetate).Acta Crystallogr E Crystallogr Commun. 2021 May 11;77(Pt 6):609-614. doi: 10.1107/S2056989021004801. eCollection 2021 Jun 1. Acta Crystallogr E Crystallogr Commun. 2021. PMID: 34164137 Free PMC article.
-
Editorial for the Special Issue: Functional Polymer Composites.Polymers (Basel). 2021 Mar 16;13(6):909. doi: 10.3390/polym13060909. Polymers (Basel). 2021. PMID: 33809448 Free PMC article.
References
-
- Skotheim T.A.E. Handbook of Conducting Polymers. Dekker; Michigan City, IN, USA: 1986.
-
- Chiang C.K., Park Y.W., Heeger A.J., Shirakawa H., Louis E.J., Gau S.C., MacDiarmid A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977;39:1098–1101. doi: 10.1103/PhysRevLett.39.1098. - DOI
-
- Hajian A., Rafati A.A., Afraz A., Najafi M. Electrosynthesis of polythiophene nanowires and their application for sensing of chlorpromazine. J. Electrochem. Soc. 2014;161:B196–B200. doi: 10.1149/2.0881409jes. - DOI
-
- English J.T., Deore B.A., Freund M.S. Biogenic amine vapor detection using poly (aniline boronic acid) films. Sens. Actuators B. 2006;115:666–671. doi: 10.1016/j.snb.2005.10.035. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

