Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2
- PMID: 32466458
- PMCID: PMC7354519
- DOI: 10.3390/v12060582
Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2
Abstract
The recent outbreak of the Coronavirus disease 2019 (COVID-19) has quickly spread worldwide since its discovery in Wuhan city, China in December 2019. A comprehensive strategy, including surveillance, diagnostics, research, clinical treatment, and development of vaccines, is urgently needed to win the battle against COVID-19. The past three unprecedented outbreaks of emerging human coronavirus infections at the beginning of the 21st century have highlighted the importance of readily available, accurate, and rapid diagnostic technologies to contain emerging and re-emerging pandemics. Real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) based assays performed on respiratory specimens remain the gold standard for COVID-19 diagnostics. However, point-of-care technologies and serologic immunoassays are rapidly emerging with high sensitivity and specificity as well. Even though excellent techniques are available for the diagnosis of symptomatic patients with COVID-19 in well-equipped laboratories; critical gaps still remain in screening asymptomatic people who are in the incubation phase of the virus, as well as in the accurate determination of live viral shedding during convalescence to inform decisions for ending isolation. This review article aims to discuss the currently available laboratory methods and surveillance technologies available for the detection of COVID-19, their performance characteristics and highlight the gaps in current diagnostic capacity, and finally, propose potential solutions. We also summarize the specifications of the majority of the available commercial kits (PCR, EIA, and POC) for laboratory diagnosis of COVID-19.
Keywords: COVID-19; SARS-CoV-2; diagnostic challenges; molecular testing; serology; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
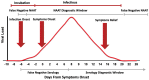
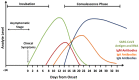
References
-
- Coronavirus Cases:. (n.d.) [(accessed on 21 May 2020)]; Available online: https://www.worldometers.info/coronavirus/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

