Crucial Role of Lamin A/C in the Migration and Differentiation of MSCs in Bone
- PMID: 32466483
- PMCID: PMC7348862
- DOI: 10.3390/cells9061330
Crucial Role of Lamin A/C in the Migration and Differentiation of MSCs in Bone
Abstract
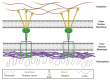
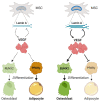
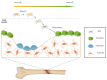
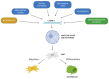
Lamin A/C, intermediate filament proteins from the nuclear lamina encoded by the LMNA gene, play a central role in mediating the mechanosignaling of cytoskeletal forces into nucleus. In fact, this mechanotransduction process is essential to ensure the proper functioning of other tasks also mediated by lamin A/C: the structural support of the nucleus and the regulation of gene expression. In this way, lamin A/C is fundamental for the migration and differentiation of mesenchymal stem cells (MSCs), the progenitors of osteoblasts, thus affecting bone homeostasis. Bone formation is a complex process regulated by chemical and mechanical cues, coming from the surrounding extracellular matrix. MSCs respond to signals modulating the expression levels of lamin A/C, and therefore, adapting their nuclear shape and stiffness. To promote cell migration, MSCs need soft nuclei with low lamin A content. Conversely, during osteogenic differentiation, lamin A/C levels are known to be increased. Several LMNA mutations present a negative impact in the migration and osteogenesis of MSCs, affecting bone tissue homeostasis and leading to pathological conditions. This review aims to describe these concepts by discussing the latest state-of-the-art in this exciting area, focusing on the relationship between lamin A/C in MSCs' function and bone tissue from both, health and pathological points of view.
Keywords: bone disease; bone formation; differentiation; lamin A/C; mechanosensing; mesenchymal stem cells (MSCs); migration.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the review.
Figures
References
-
- Shimi T., Pfleghaar K., Kojima S., Pack C.G., Solovei I., Goldman A.E., Adam S.A., Shumaker D.K., Kinjo M., Cremer T., et al. The A- and B-type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

