CUL4-DDB1-CRBN E3 Ubiquitin Ligase Regulates Proteostasis of ClC-2 Chloride Channels: Implication for Aldosteronism and Leukodystrophy
- PMID: 32466489
- PMCID: PMC7348978
- DOI: 10.3390/cells9061332
CUL4-DDB1-CRBN E3 Ubiquitin Ligase Regulates Proteostasis of ClC-2 Chloride Channels: Implication for Aldosteronism and Leukodystrophy
Abstract
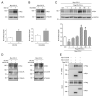
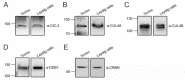
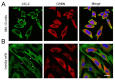
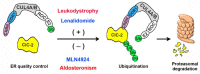
Voltage-gated ClC-2 channels are essential for chloride homeostasis. Complete knockout of mouse ClC-2 leads to testicular degeneration and neuronal myelin vacuolation. Gain-of-function and loss-of-function mutations in the ClC-2-encoding human CLCN2 gene are linked to the genetic diseases aldosteronism and leukodystrophy, respectively. The protein homeostasis (proteostasis) mechanism of ClC-2 is currently unclear. Here, we aimed to identify the molecular mechanism of endoplasmic reticulum-associated degradation of ClC-2, and to explore the pathophysiological significance of disease-associated anomalous ClC-2 proteostasis. In both heterologous expression system and native neuronal and testicular cells, ClC-2 is subject to significant regulation by cullin-RING E3 ligase-mediated polyubiquitination and proteasomal degradation. The cullin 4 (CUL4)-damage-specific DNA binding protein 1 (DDB1)-cereblon (CRBN) E3 ubiquitin ligase co-exists in the same complex with and promotes the degradation of ClC-2 channels. The CRBN-targeting immunomodulatory drug lenalidomide and the cullin E3 ligase inhibitor MLN4924 promotes and attenuates, respectively, proteasomal degradation of ClC-2. Analyses of disease-related ClC-2 mutants reveal that aldosteronism and leukodystrophy are associated with opposite alterations in ClC-2 proteostasis. Modifying CUL4 E3 ligase activity with lenalidomide and MLN4924 ameliorates disease-associated ClC-2 proteostasis abnormality. Our results highlight the significant role and therapeutic potential of CUL4 E3 ubiquitin ligase in regulating ClC-2 proteostasis.
Keywords: MG132; MLN4924; channelopathy; cullin E3 ubiquitin ligase; lenalidomide; polyubiquitination; proteasomal degradation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

