Animal Models of Metabolic Epilepsy and Epilepsy Associated Metabolic Dysfunction: A Systematic Review
- PMID: 32466498
- PMCID: PMC7345684
- DOI: 10.3390/ph13060106
Animal Models of Metabolic Epilepsy and Epilepsy Associated Metabolic Dysfunction: A Systematic Review
Abstract
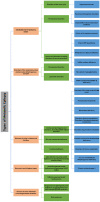
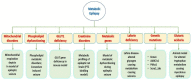
Epilepsy is a serious neurological disorder affecting around 70 million people globally and is characterized by spontaneous recurrent seizures. Recent evidence indicates that dysfunction in metabolic processes can lead to the alteration of neuronal and network excitability, thereby contributing to epileptogenesis. Developing a suitable animal model that can recapitulate all the clinical phenotypes of human metabolic epilepsy (ME) is crucial yet challenging. The specific environment of many symptoms as well as the primary state of the applicable neurobiology, genetics, and lack of valid biomarkers/diagnostic tests are the key factors that hinder the process of developing a suitable animal model. The present systematic review summarizes the current state of available animal models of metabolic dysfunction associated with epileptic disorders. A systematic search was performed by using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) model. A range of electronic databases, including google scholar, Springer, PubMed, ScienceDirect, and Scopus, were scanned between January 2000 and April 2020. Based on the selection criteria, 23 eligible articles were chosen and are discussed in the current review. Critical analysis of the selected literature delineated several available approaches that have been modeled into metabolic epilepsy and pointed out several drawbacks associated with the currently available models. The result describes available models of metabolic dysfunction associated with epileptic disorder, such as mitochondrial respiration deficits, Lafora disease (LD) model-altered glycogen metabolism, causing epilepsy, glucose transporter 1 (GLUT1) deficiency, adiponectin responsive seizures, phospholipid dysfunction, glutaric aciduria, mitochondrial disorders, pyruvate dehydrogenase (PDH) α-subunit gene (PDHA1), pyridoxine dependent epilepsy (PDE), BCL2-associated agonist of cell death (BAD), Kcna1 knock out (KO), and long noncoding RNAs (lncRNA) cancer susceptibility candidate 2 (lncRNA CASC2). Finally, the review highlights certain focus areas that may increase the possibilities of developing more suitable animal models and underscores the importance of the rationalization of animal models and evaluation methods for studying ME. The review also suggests the pressing need of developing precise robust animal models and evaluation methods for investigating ME.
Keywords: animal model; metabolic epilepsy; metabolic genes; mitochondrial dysfunction; translational research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Berg A.T., Berkovic S.F., Brodie M.J., Buchhalter J., Cross J.H., van Emde Boas W., Engel J., French J., Glauser T.A., Mathern G.W. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

