Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons
- PMID: 32466541
- PMCID: PMC7313029
- DOI: 10.3390/ijms21113756
Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons
Abstract
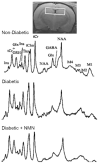
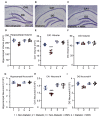
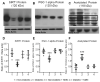
Diabetes predisposes to cognitive decline leading to dementia and is associated with decreased brain NAD+ levels. This has triggered an intense interest in boosting nicotinamide adenine dinucleotide (NAD+) levels to prevent dementia. We tested if the administration of the precursor of NAD+, nicotinamide mononucleotide (NMN), can prevent diabetes-induced memory deficits. Diabetes was induced in Sprague-Dawley rats by the administration of streptozotocin (STZ). After 3 months of diabetes, hippocampal NAD+ levels were decreased (p = 0.011). In vivo localized high-resolution proton magnetic resonance spectroscopy (MRS) of the hippocampus showed an increase in the levels of glucose (p < 0.001), glutamate (p < 0.001), gamma aminobutyric acid (p = 0.018), myo-inositol (p = 0.018), and taurine (p < 0.001) and decreased levels of N-acetyl aspartate (p = 0.002) and glutathione (p < 0.001). There was a significant decrease in hippocampal CA1 neuronal volume (p < 0.001) and neuronal number (p < 0.001) in the Diabetic rats. Diabetic rats showed hippocampal related memory deficits. Intraperitoneal NMN (100 mg/kg) was given after induction and confirmation of diabetes and was provided on alternate days for 3 months. NMN increased brain NAD+ levels, normalized the levels of glutamate, taurine, N-acetyl aspartate (NAA), and glutathione. NMN-treatment prevented the loss of CA1 neurons and rescued the memory deficits despite having no significant effect on hyperglycemic or lipidemic control. In hippocampal protein extracts from Diabetic rats, SIRT1 and PGC-1α protein levels were decreased, and acetylation of proteins increased. NMN treatment prevented the diabetes-induced decrease in both SIRT1 and PGC-1α and promoted deacetylation of proteins. Our results indicate that NMN increased brain NAD+, activated the SIRT1 pathway, preserved mitochondrial oxidative phosphorylation (OXPHOS) function, prevented neuronal loss, and preserved cognition in Diabetic rats.
Keywords: NAD+; NEDD4-1; NMN; PGC-1α; SIRT1; cognitive impairment; dementia; diabetes; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- 101RX001030/Office of Research Development, Department of Veterans Affairs
- 1R01DK107007-01A1/DK/NIDDK NIH HHS/United States
- DK072488/Atlantic Nutrition Obesity Research Center, grant P30, NIDDK, NIH
- I01 BX004895/BX/BLRD VA/United States
- BX000917 (T.K.)/Office of Research Development, Department of Veterans Affairs
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

