Effects of a Newly Developed Enzyme-Assisted Extraction Method on the Biological Activities of Fucoidans in Ocular Cells
- PMID: 32466624
- PMCID: PMC7344579
- DOI: 10.3390/md18060282
Effects of a Newly Developed Enzyme-Assisted Extraction Method on the Biological Activities of Fucoidans in Ocular Cells
Abstract
Fucoidans from brown seaweeds are promising substances as potential drugs against age-related macular degeneration (AMD). The heterogeneity of fucoidans requires intensive research in order to find suitable species and extraction methods. Ten different fucoidan samples extracted enzymatically from Laminaria digitata (LD), Saccharina latissima (SL) and Fucus distichus subsp. evanescens (FE) were tested for toxicity, oxidative stress protection and VEGF (vascular endothelial growth factor) inhibition. For this study crude fucoidans were extracted from seaweeds using different enzymes and SL fucoidans were further separated into three fractions (SL_F1-F3) by ion-exchange chromatography (IEX). Fucoidan composition was analyzed by high performance anion exchange chromatography (HPAEC) after acid hydrolysis. The crude extracts contained alginate, while two of the fractionated SL fucoidans SL_F2 and SL_F3 were highly pure. Cell viability was assessed with an 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay in OMM-1 and ARPE-19. Protective effects were investigated after 24 h of stress insult in OMM-1 and ARPE-19. Secreted VEGF was analyzed via ELISA (enzyme-linked immunosorbent assay) in ARPE-19 cells. Fucoidans showed no toxic effects. In OMM-1 SL_F2 and several FE fucoidans were protective. LD_SiAT2 (Cellic®CTec2 + Sigma-Aldrich alginate lyase), FE_SiAT3 (Cellic® CTec3 + Sigma-Aldrich alginate lyase), SL_F2 and SL_F3 inhibited VEGF with the latter two as the most effective. We could show that enzyme treated fucoidans in general and the fractionated SL fucoidans SL_F2 and SL_F3 are very promising for beneficial AMD relevant biological activities.
Keywords: Fucus distichus subsp. evanescens; Laminaria digitata; Saccharina latissima; VEGF; age-related macular degeneration; enzymatic purification; fucoidan; fucose; oxidative stress; retinal pigment epithelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


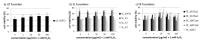


References
-
- Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
-
- Hageman G.S., Anderson D.H., Johnson L.V., Hancox L.S., Taiber A.J., Hardisty L.I., Hageman J.L., Stockman H.A., Borchardt J.D., Gehrs K.M., et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

