Retinol-binding protein 2 (RBP2): biology and pathobiology
- PMID: 32466661
- PMCID: PMC7593873
- DOI: 10.1080/10409238.2020.1768207
Retinol-binding protein 2 (RBP2): biology and pathobiology
Abstract
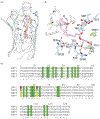
Retinol-binding protein 2 (RBP2; originally cellular retinol-binding protein, type II (CRBPII)) is a 16 kDa cytosolic protein that in the adult is localized predominantly to absorptive cells of the proximal small intestine. It is well established that RBP2 plays a central role in facilitating uptake of dietary retinoid, retinoid metabolism in enterocytes, and retinoid actions locally within the intestine. Studies of mice lacking Rbp2 establish that Rbp2 is not required in times of dietary retinoid-sufficiency. However, in times of dietary retinoid-insufficiency, the complete lack of Rbp2 gives rise to perinatal lethality owing to RBP2 absence in both placental (maternal) and neonatal tissues. Moreover, when maintained on a high-fat diet, Rbp2-knockout mice develop obesity, glucose intolerance and a fatty liver. Unexpectedly, recent investigations have demonstrated that RBP2 binds long-chain 2-monoacylglycerols (2-MAGs), including the canonical endocannabinoid 2-arachidonoylglycerol, with very high affinity, equivalent to that of retinol binding. Crystallographic studies establish that 2-MAGs bind to a site within RBP2 that fully overlaps with the retinol binding site. When challenged orally with fat, mucosal levels of 2-MAGs in Rbp2 null mice are significantly greater than those of matched controls establishing that RBP2 is a physiologically relevant MAG-binding protein. The rise in MAG levels is accompanied by elevations in circulating levels of the hormone glucose-dependent insulinotropic polypeptide (GIP). It is not understood how retinoid and/or MAG binding to RBP2 affects the functions of this protein, nor is it presently understood how these contribute to the metabolic and hormonal phenotypes observed for Rbp2-deficient mice.
Keywords: Vitamin A; endocannabinoid; enteroendocrine signaling; glucose-dependent insulinotropic polypeptide (GIP) and dietary fat; intestine; monoacylglycerol; obesity; retinoid.
Figures


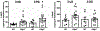
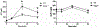
References
-
- Agace WW, Persson EK. 2012. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 33(1):42–48. - PubMed
-
- Bhat PV. 1998. Retinal dehydrogenase gene expression in stomach and small intestine of rats during postnatal development and in vitamin A deficiency. FEBS Lett. 426(2):260–262. - PubMed
-
- Blaner WS, Li Y. 2015. Vitamin A metabolism, storage and tissue delivery mechanisms In: Doll e P, Niederreither K, editors. The retinoids; biology, biochemistry, and disease. Hoboken (NJ): Wiley Blackwell; p. 3–34.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
