ProBDNF promotes sepsis-associated encephalopathy in mice by dampening the immune activity of meningeal CD4+ T cells
- PMID: 32466783
- PMCID: PMC7257240
- DOI: 10.1186/s12974-020-01850-0
ProBDNF promotes sepsis-associated encephalopathy in mice by dampening the immune activity of meningeal CD4+ T cells
Abstract
Background: Sepsis-associated encephalopathy (SAE) increases the mortality of septic patients, but its mechanism remains unclear. The present study aimed to investigate the roles of T lymphocytes, proBDNF, and their interaction in the pathogenesis of SAE.
Methods: Fear conditioning tests were conducted for cognitive assessment in the lipopolysaccharide (LPS, 5 mg kg-1)-induced septic mice. Meninges and peripheral blood were harvested for flow cytometry or qPCR. FTY720 and monoclonal anti-proBDNF antibody (McAb-proB) were used to investigate the effect of lymphocyte depletion and blocking proBDNF on the impaired cognitive functions in the septic mice.
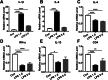
Results: In the septic mice, cognitive function was impaired, the percentage of CD4+ T cells were decreased in the meninges (P = 0.0021) and circulation (P = 0.0222), and pro-inflammatory cytokines were upregulated, but the anti-inflammatory cytokines interleukin (IL)-4 (P < 0.0001) and IL-13 (P = 0.0350) were downregulated in the meninges. Lymphocyte depletion by intragastrically treated FTY720 (1 mg kg-1) for 1 week ameliorated LPS-induced learning deficit. In addition, proBDNF was increased in the meningeal (P = 0.0042) and peripheral (P = 0.0090) CD4+ T cells. Intraperitoneal injection of McAb-proB (100 μg) before LPS treatment significantly alleviated cognitive dysfunction, inhibited the downregulation of meningeal (P = 0.0264) and peripheral (P = 0.0080) CD4+ T cells, and normalized the gene expression of cytokines in the meninges. However, intra-cerebroventricular McAb-proB injection (1 μg) did not have such effect. Finally, exogenous proBDNF downregulated the percentage of CD4+ T cells in cultured splenocytes from septic mice (P = 0.0021).
Conclusion: Upregulated proBDNF in immune system promoted the pathogenesis of SAE through downregulating the circulating CD4+ T cells, limiting its infiltration into the meninges and perturbing the meningeal pro-/anti-inflammatory homeostasis.
Keywords: Brain-derived neurotrophic factor precursor; CD4+ T cells; Encephalopathy; Meningeal immunity; Monoclonal antibody; Sepsis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









References
-
- Sonneville R, Montmollin ED, Poujade J, Garrouste-Orgeas M, Souweine B, Darmon M, Mariotte E, Argaud L, Barbier F, Goldgran-Toledano D. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 2017;43(8):1–10. doi: 10.1007/s00134-017-4807-z. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

