LKB1 mutations are not associated with the efficacy of first-line and second-line chemotherapy in patients with advanced non-small-cell lung cancer (NSCLC): a post hoc analysis of the TAILOR trial
- PMID: 32467099
- PMCID: PMC7259832
- DOI: 10.1136/esmoopen-2020-000748
LKB1 mutations are not associated with the efficacy of first-line and second-line chemotherapy in patients with advanced non-small-cell lung cancer (NSCLC): a post hoc analysis of the TAILOR trial
Abstract
Purpose: In patients with advanced lung adenocarcinoma, the impact of LKB1 mutations on cytotoxic chemotherapy efficacy remains poorly explored. Here, we aimed at investigating the potential impact of LKB1 mutational status on chemotherapy efficacy in advanced non-small-cell lung cancer (NSCLC) patients enrolled in the TArceva Italian Lung Optimisation tRial (TAILOR) trial.
Methods: The multicenter TAILOR trial randomised patients with EGFR-wild type (wt) advanced NSCLC progressing on/after previous platinum-based chemotherapy to receive docetaxel or erlotinib. Here, we evaluated the impact of LKB1 mutational status on progression-free survival (PFS) and overall survival (OS) in patients treated with second-line docetaxel/erlotinib or during prior platinum-based chemotherapy.
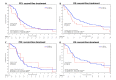
Results: Out of 222 patients randomised in the TAILOR trial, left-over tumour tissues were available for 188 patients, and 120 patients with evaluable LKB1 status were included. Of them, 17 (14.17%) patients had LKB1-mutated tumours, while 103 (85.83%) had LKB1-wt disease. During second-line treatment, PFS and OS were not statistically significantly different in patients with LKB1-mutated when compared with LKB1-wt NSCLC (adjusted HR (aHR)=1.29, 95% CI 0.75 to 2.21; p=0.364 and aHR=1.41, 95% CI 0.82 to 2.44; p=0.218, respectively). Similarly, we found no significant association between LKB1 mutations and patient PFS or OS during prior first-line platinum-based chemotherapy (aHR=1.04, 95% CI 0.55 to 1.97; p=0.910 and aHR=0.83, 95% CI 0.42 to 1.65; p=0.602, respectively).
Conclusion: Among advanced NSCLC patients receiving two lines of systemic therapy, LKB1 mutations were not associated with PFS or OS during second-line docetaxel or prior first-line platinum-based chemotherapy. While larger prospective trials are needed to confirm our findings, cytotoxic chemotherapy remains the backbone of investigational combination strategies in this patient population.
Keywords: LKB1 mutations; advanced non-small-cell lung cancer (NSCLC); chemotherapy; docetaxel; platinum chemotherapy.
© Author (s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ on behalf of the European Society for Medical Oncology.
Conflict of interest statement
Competing interests: Dr. Garassino reported personal fees from Merck, BMS, AstraZeneca, Roche, Takeda, Celgene, Pfizer, and GSK. No other disclosures were reported.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

