PI(4,5)P2-dependent regulation of exocytosis by amisyn, the vertebrate-specific competitor of synaptobrevin 2
- PMID: 32467162
- PMCID: PMC7306819
- DOI: 10.1073/pnas.1908232117
PI(4,5)P2-dependent regulation of exocytosis by amisyn, the vertebrate-specific competitor of synaptobrevin 2
Abstract
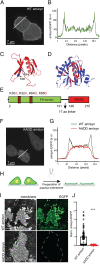
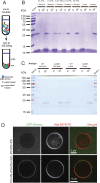
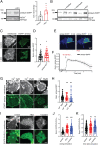
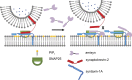
The functions of nervous and neuroendocrine systems rely on fast and tightly regulated release of neurotransmitters stored in secretory vesicles through SNARE-mediated exocytosis. Few proteins, including tomosyn (STXBP5) and amisyn (STXBP6), were proposed to negatively regulate exocytosis. Little is known about amisyn, a 24-kDa brain-enriched protein with a SNARE motif. We report here that full-length amisyn forms a stable SNARE complex with syntaxin-1 and SNAP-25 through its C-terminal SNARE motif and competes with synaptobrevin-2/VAMP2 for the SNARE-complex assembly. Furthermore, amisyn contains an N-terminal pleckstrin homology domain that mediates its transient association with the plasma membrane of neurosecretory cells by binding to phospholipid PI(4,5)P2 However, unlike synaptrobrevin-2, the SNARE motif of amisyn is not sufficient to account for the role of amisyn in exocytosis: Both the pleckstrin homology domain and the SNARE motif are needed for its inhibitory function. Mechanistically, amisyn interferes with the priming of secretory vesicles and the sizes of releasable vesicle pools, but not vesicle fusion properties. Our biochemical and functional analyses of this vertebrate-specific protein unveil key aspects of negative regulation of exocytosis.
Keywords: PI(4,5)P2; SNARE complex; autism spectrum disorders; exocytosis inhibition; tomosyn.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Amisyn regulates exocytosis and fusion pore stability by both syntaxin-dependent and syntaxin-independent mechanisms.J Biol Chem. 2005 Sep 9;280(36):31615-23. doi: 10.1074/jbc.M505858200. Epub 2005 Jul 20. J Biol Chem. 2005. PMID: 16033762
-
Amisyn, a novel syntaxin-binding protein that may regulate SNARE complex assembly.J Biol Chem. 2002 Aug 2;277(31):28271-9. doi: 10.1074/jbc.M204929200. Epub 2002 May 24. J Biol Chem. 2002. PMID: 12145319
-
The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis.J Biol Chem. 2003 Aug 15;278(33):31159-66. doi: 10.1074/jbc.M305500200. Epub 2003 Jun 2. J Biol Chem. 2003. PMID: 12782620
-
Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion.Subcell Biochem. 2012;59:111-30. doi: 10.1007/978-94-007-3015-1_4. Subcell Biochem. 2012. PMID: 22374089 Free PMC article. Review.
-
PI(4,5)P₂-binding effector proteins for vesicle exocytosis.Biochim Biophys Acta. 2015 Jun;1851(6):785-93. doi: 10.1016/j.bbalip.2014.09.017. Epub 2014 Oct 2. Biochim Biophys Acta. 2015. PMID: 25280637 Free PMC article. Review.
Cited by
-
Control of synaptic vesicle release probability via VAMP4 targeting to endolysosomes.Sci Adv. 2021 Apr 30;7(18):eabf3873. doi: 10.1126/sciadv.abf3873. Print 2021 Apr. Sci Adv. 2021. PMID: 33931449 Free PMC article.
-
Alterations of presynaptic proteins in autism spectrum disorder.Front Mol Neurosci. 2022 Nov 17;15:1062878. doi: 10.3389/fnmol.2022.1062878. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36466804 Free PMC article. Review.
-
STXBP6 Gene Mutation: A New Form of SNAREopathy Leads to Developmental Epileptic Encephalopathy.Int J Mol Sci. 2023 Nov 17;24(22):16436. doi: 10.3390/ijms242216436. Int J Mol Sci. 2023. PMID: 38003627 Free PMC article.
-
Whole Exome Sequencing as a First-Line Molecular Genetic Test in Developmental and Epileptic Encephalopathies.Int J Mol Sci. 2024 Jan 17;25(2):1146. doi: 10.3390/ijms25021146. Int J Mol Sci. 2024. PMID: 38256219 Free PMC article.
-
Behavioral and Gene Expression Analysis of Stxbp6-Knockout Mice.Brain Sci. 2021 Mar 29;11(4):436. doi: 10.3390/brainsci11040436. Brain Sci. 2021. PMID: 33805317 Free PMC article.
References
-
- Terrian D. M., White M. K., Phylogenetic analysis of membrane trafficking proteins: A family reunion and secondary structure predictions. Eur. J. Cell Biol. 73, 198–204 (1997). - PubMed
-
- Weimbs T., Mostov K., Low S. H., Hofmann K., A model for structural similarity between different SNARE complexes based on sequence relationships. Trends Cell Biol. 8, 260–262 (1998). - PubMed
-
- Sutton R. B., Fasshauer D., Jahn R., Brunger A. T., Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998). - PubMed
-
- Fasshauer D., Eliason W. K., Brünger A. T., Jahn R., Identification of a minimal core of the synaptic SNARE complex sufficient for reversible assembly and disassembly. Biochemistry 37, 10354–10362 (1998). - PubMed
-
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E., A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409–418 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

