PI(4,5)P2-dependent regulation of exocytosis by amisyn, the vertebrate-specific competitor of synaptobrevin 2
- PMID: 32467162
- PMCID: PMC7306819
- DOI: 10.1073/pnas.1908232117
PI(4,5)P2-dependent regulation of exocytosis by amisyn, the vertebrate-specific competitor of synaptobrevin 2
Abstract
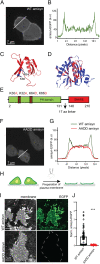
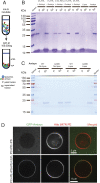
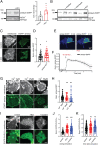
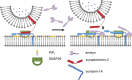
The functions of nervous and neuroendocrine systems rely on fast and tightly regulated release of neurotransmitters stored in secretory vesicles through SNARE-mediated exocytosis. Few proteins, including tomosyn (STXBP5) and amisyn (STXBP6), were proposed to negatively regulate exocytosis. Little is known about amisyn, a 24-kDa brain-enriched protein with a SNARE motif. We report here that full-length amisyn forms a stable SNARE complex with syntaxin-1 and SNAP-25 through its C-terminal SNARE motif and competes with synaptobrevin-2/VAMP2 for the SNARE-complex assembly. Furthermore, amisyn contains an N-terminal pleckstrin homology domain that mediates its transient association with the plasma membrane of neurosecretory cells by binding to phospholipid PI(4,5)P2 However, unlike synaptrobrevin-2, the SNARE motif of amisyn is not sufficient to account for the role of amisyn in exocytosis: Both the pleckstrin homology domain and the SNARE motif are needed for its inhibitory function. Mechanistically, amisyn interferes with the priming of secretory vesicles and the sizes of releasable vesicle pools, but not vesicle fusion properties. Our biochemical and functional analyses of this vertebrate-specific protein unveil key aspects of negative regulation of exocytosis.
Keywords: PI(4,5)P2; SNARE complex; autism spectrum disorders; exocytosis inhibition; tomosyn.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Terrian D. M., White M. K., Phylogenetic analysis of membrane trafficking proteins: A family reunion and secondary structure predictions. Eur. J. Cell Biol. 73, 198–204 (1997). - PubMed
-
- Weimbs T., Mostov K., Low S. H., Hofmann K., A model for structural similarity between different SNARE complexes based on sequence relationships. Trends Cell Biol. 8, 260–262 (1998). - PubMed
-
- Sutton R. B., Fasshauer D., Jahn R., Brunger A. T., Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998). - PubMed
-
- Fasshauer D., Eliason W. K., Brünger A. T., Jahn R., Identification of a minimal core of the synaptic SNARE complex sufficient for reversible assembly and disassembly. Biochemistry 37, 10354–10362 (1998). - PubMed
-
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E., A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409–418 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

