Anisotropy links cell shapes to tissue flow during convergent extension
- PMID: 32467168
- PMCID: PMC7306759
- DOI: 10.1073/pnas.1916418117
Anisotropy links cell shapes to tissue flow during convergent extension
Abstract
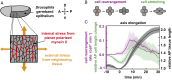
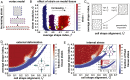
Within developing embryos, tissues flow and reorganize dramatically on timescales as short as minutes. This includes epithelial tissues, which often narrow and elongate in convergent extension movements due to anisotropies in external forces or in internal cell-generated forces. However, the mechanisms that allow or prevent tissue reorganization, especially in the presence of strongly anisotropic forces, remain unclear. We study this question in the converging and extending Drosophila germband epithelium, which displays planar-polarized myosin II and experiences anisotropic forces from neighboring tissues. We show that, in contrast to isotropic tissues, cell shape alone is not sufficient to predict the onset of rapid cell rearrangement. From theoretical considerations and vertex model simulations, we predict that in anisotropic tissues, two experimentally accessible metrics of cell patterns-the cell shape index and a cell alignment index-are required to determine whether an anisotropic tissue is in a solid-like or fluid-like state. We show that changes in cell shape and alignment over time in the Drosophila germband predict the onset of rapid cell rearrangement in both wild-type and snail twist mutant embryos, where our theoretical prediction is further improved when we also account for cell packing disorder. These findings suggest that convergent extension is associated with a transition to more fluid-like tissue behavior, which may help accommodate tissue-shape changes during rapid developmental events.
Keywords: Drosophila; epithelia; morphogenesis; vertex models.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Keller R., Developmental biology. Physical biology returns to morphogenesis. Science 338, 201–203 (2012). - PubMed
-
- Gilmour D., Rembold M., Leptin M., From morphogen to morphogenesis and back. Nature 541, 311–320 (2017). - PubMed
-
- Davidson L. A., Embryo mechanics: Balancing force production with elastic resistance during morphogenesis. Curr. Top. Dev. Biol. 95, 215–241 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

