Coagulopathy of Coronavirus Disease 2019
- PMID: 32467443
- PMCID: PMC7255402
- DOI: 10.1097/CCM.0000000000004458
Coagulopathy of Coronavirus Disease 2019
Abstract
Objectives: Recent studies have reported a high prevalence of thrombotic events in coronavirus disease 2019. However, the significance of thromboembolic complications has not been widely appreciated. The purpose of this review is to provide current knowledge of this serious problem.
Design: Narrative review.
Data sources: Online search of published medical literature through PubMed using the term "COVID-19," "SARS," "acute respiratory distress syndrome," "coronavirus," "coagulopathy," "thrombus," and "anticoagulants."
Study selection and data extraction: Articles were chosen for inclusion based on their relevance to coagulopathy and thrombosis in coronavirus disease 2019, and anticoagulant therapy. Reference lists were reviewed to identify additional relevant articles.
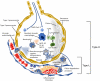
Data synthesis: Coronavirus disease 2019 is associated with a strikingly high prevalence of coagulopathy and venous thromboembolism that may contribute to respiratory deterioration. Monitoring coagulation variables is important, as abnormal coagulation tests are related to adverse outcomes and may necessitate adjuvant antithrombotic interventions. In the initial phase of the infection, D-dimer and fibrinogen levels are increased, while activated partial prothrombin time, prothrombin time, and platelet counts are often relatively normal. Increased D-dimer levels three times the upper limit of normal may trigger screening for venous thromboembolism. In all hospitalized patients, thromboprophylaxis using low-molecular-weight heparin is currently recommended. The etiology of the procoagulant responses is complex and thought to be a result of specific interactions between host defense mechanisms and the coagulation system. Although the coagulopathy is reminiscent of disseminated intravascular coagulation and thrombotic microangiopathy, it has features that are markedly distinct from these entities.
Conclusions: Severe acute respiratory syndrome coronavirus 2/coronavirus disease 2019 frequently induces hypercoagulability with both microangiopathy and local thrombus formation, and a systemic coagulation defect that leads to large vessel thrombosis and major thromboembolic complications, including pulmonary embolism in critically ill hospitalized patients. D-dimers and fibrinogen levels should be monitored, and all hospitalized patients should undergo thromboembolism prophylaxis with an increase in therapeutic anticoagulation in certain clinical situations.
Conflict of interest statement
Dr. Levy received funding from research, data safety, or advisory committees for CSL Behring, Instrumentation Labs, Janssen, Merck, and Octapharma. Dr. Connors’ institution received funding from CSL Behring; received funding from Abbott (consulting), Bristol-Myers Squibb (consulting), and Portola (scientific advisory board). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

Comment in
-
Optimizing the Risk-Benefit Balance of Thromboprophylaxis in Critically Ill Patients With Coronavirus Disease 2019.Crit Care Med. 2020 Oct;48(10):e988-e989. doi: 10.1097/CCM.0000000000004509. Crit Care Med. 2020. PMID: 32706558 Free PMC article. No abstract available.
-
In Response to "Coagulopathy of Coronavirus Disease 2019".Crit Care Med. 2020 Nov;48(11):e1159-e1160. doi: 10.1097/CCM.0000000000004545. Crit Care Med. 2020. PMID: 32897666 Free PMC article. No abstract available.
-
The authors reply.Crit Care Med. 2020 Oct;48(10):e989-e990. doi: 10.1097/CCM.0000000000004530. Crit Care Med. 2020. PMID: 32931200 Free PMC article. No abstract available.
-
The authors reply.Crit Care Med. 2020 Nov;48(11):e1160-e1161. doi: 10.1097/CCM.0000000000004616. Crit Care Med. 2020. PMID: 32932349 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

