Nanomedicine-based immunotherapy for central nervous system disorders
- PMID: 32467570
- PMCID: PMC7468531
- DOI: 10.1038/s41401-020-0429-z
Nanomedicine-based immunotherapy for central nervous system disorders
Abstract
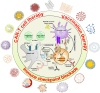
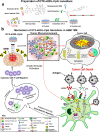
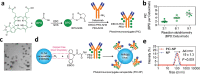
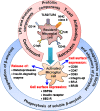
Central nervous system (CNS) disorders represent a broad spectrum of brain ailments with short- and long-term disabilities, and nanomedicine-based approaches provide a new therapeutic approach to treating CNS disorders. A variety of potential drugs have been discovered to treat several neuronal disorders; however, their therapeutic success can be limited by the presence of the blood-brain barrier (BBB). Furthermore, unique immune functions within the CNS provide novel target mechanisms for the amelioration of CNS diseases. Recently, various therapeutic approaches have been applied to fight brain-related disorders, with moderate outcomes. Among the various therapeutic strategies, nanomedicine-based immunotherapeutic systems represent a new era that can deliver useful cargo with promising pharmacokinetics. These approaches exploit the molecular and cellular targeting of CNS disorders for enhanced safety, efficacy, and specificity. In this review, we focus on the efficacy of nanomedicines that utilize immunotherapy to combat CNS disorders. Furthermore, we detailed summarize nanomedicine-based pathways for CNS ailments that aim to deliver drugs across the BBB by mimicking innate immune actions. Overview of how nanomedicines can utilize multiple immunotherapy pathways to combat CNS disorders.
Keywords: blood–brain barrier; central nervous system disorders; immunotherapy; nanomedicine.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Role of Nanomedicine-Based Therapeutics in the Treatment of CNS Disorders.Molecules. 2023 Jan 28;28(3):1283. doi: 10.3390/molecules28031283. Molecules. 2023. PMID: 36770950 Free PMC article. Review.
-
Targeting specific cells in the brain with nanomedicines for CNS therapies.J Control Release. 2016 Oct 28;240:212-226. doi: 10.1016/j.jconrel.2015.12.013. Epub 2015 Dec 11. J Control Release. 2016. PMID: 26686078 Free PMC article. Review.
-
Delivering the Promise of Gene Therapy with Nanomedicines in Treating Central Nervous System Diseases.Adv Sci (Weinh). 2022 Sep;9(26):e2201740. doi: 10.1002/advs.202201740. Epub 2022 Jul 18. Adv Sci (Weinh). 2022. PMID: 35851766 Free PMC article. Review.
-
ROS Regulation in CNS Disorder Therapy: Unveiling the Dual Roles of Nanomedicine.Small. 2025 Feb;21(5):e2410031. doi: 10.1002/smll.202410031. Epub 2024 Dec 15. Small. 2025. PMID: 39676433 Review.
-
Imaging and nanomedicine for diagnosis and therapy in the central nervous system: report of the eleventh annual Blood-Brain Barrier Disruption Consortium meeting.AJNR Am J Neuroradiol. 2006 Mar;27(3):715-21. AJNR Am J Neuroradiol. 2006. PMID: 16552023 Free PMC article.
Cited by
-
Nanotechnological approaches for pentamidine delivery.Drug Deliv Transl Res. 2022 Aug;12(8):1911-1927. doi: 10.1007/s13346-022-01127-4. Epub 2022 Feb 25. Drug Deliv Transl Res. 2022. PMID: 35217992 Free PMC article. Review.
-
Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy.Acta Pharmacol Sin. 2022 Nov;43(11):2749-2758. doi: 10.1038/s41401-022-00910-w. Epub 2022 Apr 28. Acta Pharmacol Sin. 2022. PMID: 35484402 Free PMC article. Review.
-
Investigating the Potential Therapeutic Mechanisms of Puerarin in Neurological Diseases.Mol Neurobiol. 2024 Dec;61(12):10747-10769. doi: 10.1007/s12035-024-04222-4. Epub 2024 May 23. Mol Neurobiol. 2024. PMID: 38780722 Review.
-
Risk Factors, Outcomes, and Predictions of Extensively Drug-Resistant Acinetobacter baumannii Nosocomial Infections in Patients with Nervous System Diseases.Infect Drug Resist. 2023 Nov 22;16:7327-7337. doi: 10.2147/IDR.S439241. eCollection 2023. Infect Drug Resist. 2023. PMID: 38023397 Free PMC article.
-
Brain-on-a-chip: an emerging platform for studying the nanotechnology-biology interface for neurodegenerative disorders.J Nanobiotechnology. 2024 Sep 18;22(1):573. doi: 10.1186/s12951-024-02720-0. J Nanobiotechnology. 2024. PMID: 39294645 Free PMC article. Review.
References
-
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Zanganeh S, Georgala P, Corbo C, Arabi L, Ho JQ, Javdani N, et al. Immunoengineering in glioblastoma imaging and therapy. Wiley Interdiscip Rev. 2019;11:1575. - PubMed
-
- Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–37. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

