Biallelic PDE2A variants: a new cause of syndromic paroxysmal dyskinesia
- PMID: 32467598
- PMCID: PMC7608189
- DOI: 10.1038/s41431-020-0641-9
Biallelic PDE2A variants: a new cause of syndromic paroxysmal dyskinesia
Abstract
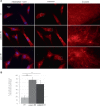
Cause of complex dyskinesia remains elusive in some patients. A homozygous missense variant leading to drastic decrease of PDE2A enzymatic activity was reported in one patient with childhood-onset choreodystonia preceded by paroxysmal dyskinesia and associated with cognitive impairment and interictal EEG abnormalities. Here, we report three new cases with biallelic PDE2A variants identified by trio whole-exome sequencing. Mitochondria network was analyzed after Mitotracker™ Red staining in control and mutated primary fibroblasts. Analysis of retrospective video of patients' movement disorder and refinement of phenotype was carried out. We identified a homozygous gain of stop codon variant c.1180C>T; p.(Gln394*) in PDE2A in siblings and compound heterozygous variants in young adult: a missense c.446C>T; p.(Pro149Leu) and splice-site variant c.1922+5G>A predicted and shown to produce an out of frame transcript lacking exon 22. All three patients had cognitive impairment or developmental delay. The phenotype of the two oldest patients, aged 9 and 26, was characterized by childhood-onset refractory paroxysmal dyskinesia initially misdiagnosed as epilepsy due to interictal EEG abnormalities. The youngest patient showed a proven epilepsy at the age of 4 months and no paroxysmal dyskinesia at 15 months. Interestingly, analysis of the fibroblasts with the biallelic variants in PDE2A variants revealed mitochondria network morphology changes. Together with previously reported case, our three patients confirm that biallelic PDE2A variants are a cause of childhood-onset refractory paroxysmal dyskinesia with cognitive impairment, sometimes associated with choreodystonia and interictal baseline EEG abnormalities or epilepsy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

