A comparison of biologicals in the treatment of adults with severe asthma - real-life experiences
- PMID: 32467765
- PMCID: PMC7222440
- DOI: 10.1186/s40733-020-00055-9
A comparison of biologicals in the treatment of adults with severe asthma - real-life experiences
Erratum in
-
Correction to: A comparison of biologicals in the treatment of adults with severe asthma - real-life experiences.Asthma Res Pract. 2020 Oct 2;6:10. doi: 10.1186/s40733-020-00063-9. eCollection 2020. Asthma Res Pract. 2020. PMID: 33024570 Free PMC article.
Abstract
Background: Anti-IgE (omalizumab) and anti-IL5/IL5R (reslizumab, mepolizumab and benralizumab) treatments are available for severe allergic and eosinophilic asthma. In these patients, studies have shown beneficial effects in oral corticosteroid use and exacerbations. The aim of this retrospective single-center study was to evaluate the effect of biological therapy on severe asthma and to compare different therapies.
Methods: We collected and analysed results of anti-IL5/IL5R and anti-IgE therapies for asthma from January 2009 until October 2019 in specialized care. We compared number of exacerbations, asthma symptoms and use of per oral corticosteroids and antimicrobics because of asthma before and during biological therapy, and in a separate analysis need for per oral corticosteroids, antimicrobics or surgery due to upper respiratory tract diseases in asthmatics receiving biologicals. The analyses were done using the Chi square test, T-test or Mann-Whitney U -test, the Kruskall-Wallis test or the Wilcoxon test.
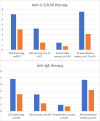
Results: Of 64 patients, 40 used continuous per oral corticosteroid therapy prior to biological therapy. The mean daily dose of per oral corticosteroid was reduced in those with anti-IL5/IL5R therapy (- 3.0 mg, p = 0.02). The number of annual per oral corticosteroid courses decreased in both the anti-IL5/IL5R (- 2.8 courses, p < 0.05) and anti-IgE groups (- 1.3 courses, p < 0.05). The number of annual antibiotic courses (- 0.7 courses, p = 0.04) and total number of exacerbation events (- 4.4 events/year, p < 0.05) were reduced in the anti-IL5/IL5R group. In the 55 asthma patients analysed for upper respiratory tract findings, the results suggested a reduction in need for chronic rhinosinusitis surgery during biological therapy.
Conclusions: Results with biological therapies in this real-life clinical setting are comparable to those reported in clinical trials. Biological therapy reduces exacerbations and per oral corticosteroid use.
Trial registration: NCT04158050, retrospectively registered 6.11.2019.
Keywords: Anti-IL5; Anti-IgE; Asthma; Biological therapy; Chronic rhinosinusitis; Corticosteroid; Eosinophils; Exacerbation; IgE.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests concerning this work. Outside this work, Emma Kotisalmi has received a personal fee from the Finnish doctors’ society Duodecim for a manuscript and an abroad congress fee from Orion Pharma. Outside this work, Paula Kauppi has received personal fees from Novartis, TEVA, and GSK for lecturing, personal fees from Sanofi and The Finnish Work Environment Fund for consultancy and a personal fee from Fimea for a manuscript. Outside this work, Sanna Toppila-Salmi has received personal fees from Mylan Laboratories Ltd., Biomedical systems Ltd., Roche Products Ltd., Sanofi Pharma Ltd. and Novartis Investments for consultancy.
Figures
References
-
- Global Initiative for asthma . Global Strategy for Asthma Management and Prevention. 2019.
-
- Chung F, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. Erratum: “International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma”. Eur Respir J. 2018;52(1):13993003. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

