Fusarium volatile, a new potential pathogen from a human respiratory sample
- PMID: 32467910
- PMCID: PMC7241678
- DOI: 10.3114/fuse.2019.04.09
Fusarium volatile, a new potential pathogen from a human respiratory sample
Abstract
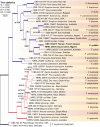
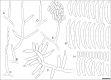
We describe the isolation and characterization of Fusarium volatile from a bronchoalveolar lavage (BAL) sample of a female patient living in French Guiana with underlying pulmonary infections. Phylogenetic analysis of fragments of the calmodulin (cmdA), translation elongation factor (tef1), RNA polymerase second largest subunit (rpb2), and β-tubulin (tub) loci revealed that strain CBS 143874 was closely related to isolate NRRL 25615, a known but undescribed phylogenetic species belonging to the African clade of the Fusarium fujikuroi species complex. The fungus differed phylogenetically and morphologically from related known species, and is therefore described as the new taxon Fusarium volatile. Antifungal susceptibility testing suggested that the new species is resistant to echinocandins, fluconazole, itraconazole with lower MICs against amphotericin B, voriconazole and posaconazole.
Keywords: FFSC; French Guiana; clinical samples; fungal taxonomy; phylogeny.
© 2019 Westerdijk Fungal Biodiversity Institute.
Figures


References
-
- Al-Hatmi AM, Bonifaz A, Ranque R, et al. (2017). Current antifungal treatment of fusariosis. International Journal of Antimicrobial 51: 326–332. - PubMed
-
- Al-Hatmi AM, van Diepeningen AD, Curfs-Breuker I, et al. (2015). Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. Journal of Antimicrobial Chemotherapy 70: 1068–1071. - PubMed
-
- Aoki T, Smith JA, Mount LL, et al. (2013). Fusarium torreyae sp. nov., a pathogen causing canker disease of Florida torreya (Torreya taxifolia), a critically endangered conifer restricted to northern Florida and southwestern Georgia. Mycologia 105: 312–319. - PubMed
LinkOut - more resources
Full Text Sources
