Hyperoxia reduces STX17 expression and inhibits the autophagic flux in alveolar type II epithelial cells in newborn rats
- PMID: 32467992
- PMCID: PMC7307846
- DOI: 10.3892/ijmm.2020.4617
Hyperoxia reduces STX17 expression and inhibits the autophagic flux in alveolar type II epithelial cells in newborn rats
Abstract
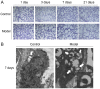
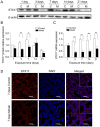
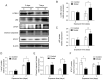
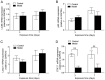
Supplemental oxygen therapy can be life‑saving for premature infants. Our previous study revealed a defect in the autophagic flux in the lung tissues of neonatal rats with hyperoxia‑induced bronchopulmonary dysplasia (BPD), but the underlying mechanism remains unknown. Moreover, there are few innovative treatments that can completely alter the course of BPD. The present study examined the expression of Syntaxin 17 (STX17), a protein necessary for autophagosome‑lysosome binding, in alveolar type II (AT‑II) epithelial cells of neonatal rats with BPD. Neonatal Sprague‑Dawley rats were randomly exposed to elevated O2 levels [fraction of inspired oxygen (FiO2), 0.8; model group] or normal room air (FiO2, 0.21; control group), and the expression levels of STX17, autophagy‑related [Microtubule‑associated protein 1A/1B‑light chain 3B (LC3B)‑II, p62, lysosomal‑associated membrane protein 1)] and apoptosis‑related (cleaved caspase3) mRNA and proteins were examined in lung tissues. Moreover, the expression levels of the aforementioned proteins were measured in isolated primary AT‑II cells cultured in vitro under hyperoxic conditions in the presence or absence of pharmacological modulators of autophagy. Transmission electron microscopy identified that AT‑II cell apoptosis and autophagosome aggregation were elevated in the lungs of BPD rats compared with control rats on postnatal day 7. STX17 mRNA and protein expression levels were decreased in lung tissue and isolated AT‑II cells as early as postnatal day 3 in BPD rats, while the expression levels of LC3B‑II, p62 and cleaved caspase3 were increased, reaching a peak on postnatal day 7. This early reduction in STX17 expression, followed by increased expression in autophagy‑ and apoptosis‑related proteins, was also observed in isolated AT‑II cells exposed to hyperoxia in vitro. However, treatment with the autophagy inducers rapamycin or LiCl eliminated the hyperoxia‑induced reduction in STX17, partially restored the autophagy flux and increased the survival of AT‑II cells exposed to hyperoxia. Collectively, these results indicated that STX17 expression in AT‑II cells was reduced in the early stages of BPD in neonatal rats and may be related to the subsequent hyperoxia‑induced block in autophagic flux.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

