Comprehensive analysis of the differential expression profile of microRNAs in rats with spinal cord injury treated by electroacupuncture
- PMID: 32468009
- PMCID: PMC7339738
- DOI: 10.3892/mmr.2020.11161
Comprehensive analysis of the differential expression profile of microRNAs in rats with spinal cord injury treated by electroacupuncture
Abstract
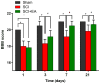
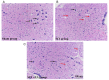
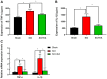
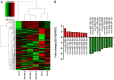
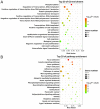
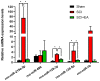
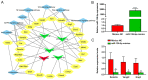
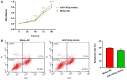
Abnormal microRNA (miRNA) expression has been implicated in spinal cord injury (SCI), but the underlying mechanisms are poorly understood. To observe the effect of electroacupuncture (EA) on miRNA expression profiles in SCI rats and investigate the potential mechanisms involved in this process, Sprague‑Dawley rats were divided into sham, SCI and SCI+EA groups (n=6 each). Basso, Beattie and Bresnahan (BBB) scoring and hematoxylin‑eosin staining of cortical tissues were used to evaluate spinal cord recovery with EA treatment 21 days post‑surgery across the three groups. To investigate miRNA expression profiles, 6 Sprague‑Dawley rats were randomly divided into SCI and SCI+EA groups (n=3 in each group) and examined using next‑generation sequencing. Integrated miRNA‑mRNA‑pathway network analysis was performed to elucidate the interaction network of the candidate miRNAs, their target genes and the involved pathways. Behavioral scores suggested that hindlimb motor functions improved with EA treatments. Apoptotic indices were lower in the SCI+EA group compared with the SCI group. It was also observed that 168 miRNAs were differentially expressed between the SCI and SCI+EA groups, with 29 upregulated and 139 downregulated miRNAs in the SCI+EA group. Changes in miRNA expression are involved in SCI physiopathology, including inflammation and apoptosis. Reverse transcription‑quantitative PCR measurement of the five candidate miRNAs, namely rno‑miR‑219a‑5p, rno‑miR‑486, rno‑miR‑136‑5p, rno‑miR‑128‑3p, and rno‑miR‑7b, was consistent with RNA sequencing data. Integrated miRNA‑mRNA‑pathway analysis suggested that the MAPK, Wnt and NF‑κB signaling pathways were involved in EA‑mediated recovery from SCI. The present study evaluated the miRNA expression profiles involved in EA‑treated SCI rats and demonstrated the potential mechanism and functional role of miRNAs in SCI in rats.
Keywords: next-generation sequencing; microrna profile; spinal cord injury; electroacupuncture; reverse transcription-qPcr.
Figures
Similar articles
-
Recovery of spinal cord injury following electroacupuncture in rats through enhancement of Wnt/β-catenin signaling.Mol Med Rep. 2017 Aug;16(2):2185-2190. doi: 10.3892/mmr.2017.6801. Epub 2017 Jun 19. Mol Med Rep. 2017. PMID: 28627669
-
[Electroacupuncture reduces neuronal apoptosis in rats with spinal cord injury by regulating GSK-3β expression and its phosphorylation].Zhen Ci Yan Jiu. 2024 Nov 25;49(11):1146-1152. doi: 10.13702/j.1000-0607.20230529. Zhen Ci Yan Jiu. 2024. PMID: 39557430 Chinese.
-
[Electroacupuncture improves limb motor function by down-regulating cytosolic phospholi-pase A2 and reducing apoptosis of nerve cells in rats with spinal cord injury].Zhen Ci Yan Jiu. 2023 Aug 25;48(8):782-90. doi: 10.13702/j.1000-0607.20220513. Zhen Ci Yan Jiu. 2023. PMID: 37614136 Chinese.
-
Research progress on the regulatory role of microRNAs in spinal cord injury.Regen Med. 2021 May;16(5):465-476. doi: 10.2217/rme-2020-0125. Epub 2021 May 6. Regen Med. 2021. PMID: 33955796 Review.
-
microRNAs in spinal cord injury: potential roles and therapeutic implications.Int J Biol Sci. 2014 Sep 6;10(9):997-1006. doi: 10.7150/ijbs.9058. eCollection 2014. Int J Biol Sci. 2014. PMID: 25210498 Free PMC article. Review.
Cited by
-
Electroacupuncture-Regulated miR-34a-3p/PDCD6 Axis Promotes Post-Spinal Cord Injury Recovery in Both In Vitro and In Vivo Settings.J Immunol Res. 2022 Sep 12;2022:9329494. doi: 10.1155/2022/9329494. eCollection 2022. J Immunol Res. 2022. PMID: 36132985 Free PMC article.
-
Electroacupuncture-Modulated MiR-106b-5p Expression Enhances Autophagy by Targeting Beclin-1 to Promote Motor Function Recovery After Spinal Cord Injury in Rats.Neurospine. 2023 Sep;20(3):1011-1027. doi: 10.14245/ns.2346446.223. Epub 2023 Aug 7. Neurospine. 2023. PMID: 37562442 Free PMC article.
-
Mechanism Underlying Acupuncture Therapy in Spinal Cord Injury: A Narrative Overview of Preclinical Studies.Front Pharmacol. 2022 Apr 7;13:875103. doi: 10.3389/fphar.2022.875103. eCollection 2022. Front Pharmacol. 2022. PMID: 35462893 Free PMC article. Review.
-
Circulating exosomal lncRNA contributes to the pathogenesis of spinal cord injury in rats.Neural Regen Res. 2023 Apr;18(4):889-894. doi: 10.4103/1673-5374.353504. Neural Regen Res. 2023. PMID: 36204859 Free PMC article.
-
Electroacupuncture protective effects after cerebral ischemia are mediated through miR-219a inhibition.Biol Res. 2023 Jun 30;56(1):36. doi: 10.1186/s40659-023-00448-z. Biol Res. 2023. PMID: 37391839 Free PMC article.
References
-
- Sharif-Alhoseini M, Rahimi-Movaghar V. Animal models in traumatic spinal cord injury. Top Paraplegia. 2014
-
- Gao L, Sun Y, Li J, Bai F, Li P. Effects of electroacupuncture in different time on variations of fractional anisotropy mean value of diffusion tensor tractogra-phy in spinal cord injured rats. Chin J Rehabil Theory Prac. 2014;20:728–733.
-
- Majdan M, Plancikova D, Nemcovska E, Krajcovicova L, Brazinova A, Rusnak M. Mortality due to traumatic spinal cord injuries in Europe: A cross-sectional and pooled analysis of population-wide data from 22 countries. Scand J Trauma Resusc Emerg Med. 2017;25:64. doi: 10.1186/s13049-017-0410-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

