MicroRNA‑93 contributes to the suppression of lung inflammatory responses in LPS‑induced acute lung injury in mice via the TLR4/MyD88/NF‑κB signaling pathway
- PMID: 32468034
- PMCID: PMC7307825
- DOI: 10.3892/ijmm.2020.4610
MicroRNA‑93 contributes to the suppression of lung inflammatory responses in LPS‑induced acute lung injury in mice via the TLR4/MyD88/NF‑κB signaling pathway
Abstract
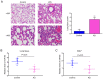
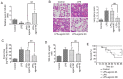
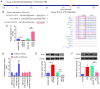
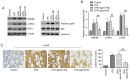
Acute lung injury (ALI) is a severe inflammatory lung disease with a rapid onset. The anti‑inflammatory functions of microRNA‑93 (miRNA/miR‑93) have been described in various types of tissue injury and disease. However, the biological role of miR‑93 and its molecular mechanisms underlying the initiation and progression of ALI have not yet been reported, at least to the best of our knowledge. The present study aimed to investigate the regulatory effects exerted by miR‑93 in ALI. Using an in vivo murine model of ALI induced by lipopolysaccharide (LPS), miR‑93 expression was found to be downregulated in the lung tissues and bronchoalveolar lavage fluid (BALF) compared with the control group. Following agomiR‑93 injection, it was observed that agomiR‑93 attenuated lung injury, as evidenced by decreased lung permeability, a reduced lung wet/dry weight ratio and an increased survival rate of the mice. Concomitantly, agomiR‑93 significantly reduced LPS‑induced the interleukin (IL)‑6, IL‑1β, and tumor necrosis factor (TNF)‑α levels in BALF. Of note, Toll‑like receptor 4 (TLR4), an upstream regulator of the nuclear factor (NF)‑κB signaling pathway, was directly suppressed by miR‑93 in RAW 264.7 cells. Importantly, agomiR‑93 induced a significant suppression of the TLR4/myeloid differentiation primary response 88 (MyD88)/NF‑κB signaling pathway, as demonstrated by the downregulation of MyD88, and the phosphorylation of IκB‑α and p65 in the lung tissues of mice with ALI. Taken together, the findings of the present study indicate that miR‑93 attenutes LPS‑induced lung injury by regulating the TLR4/MyD88/NF‑κB signaling pathway, suggesting that miR‑93 may prove to be a potential therapeutic target for ALI.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

